Introduction
The pet population in Korea continues to grow, and a survey in 2019 reported that dogs accounted for 6 million of the total 10 million pets [1]. Consequently, there has been an increase in the interest in dog healthcare and related markets. Periodontal disease is a common disease in dogs, and dental care ranked 12th among the 20 major reasons for dogs visiting private veterinary clinics in Jeollabuk-do [2].
Periodontal disease is an infectious and inflammatory disease caused by microorganisms, including bacteria, viruses, fungi, and protozoa, that colonize the tooth surfaces below the gingival margins [3,4]. The microorganisms accumulate on the surface of the teeth to form a biofilm called plaque, which persists on the surface of the teeth, thickens, and becomes tartar when mineralized [5,6]. Plaque accumulation is one of the major factors of periodontitis development in dogs, because it releases enzymes derived from a specific pathogenic bacterium–host interaction that leads to the destruction of the supporting periodontium and alveolar bone around the teeth. This finally causes loss of attachment of the teeth [7]. Moreover, plaque is extremely common in dogs because the temperature and humidity in their oral cavity is appropriate for the growth of the microorganisms, providing an ideal environment for microbial life [8].
Plaque is formed by a variety of microorganisms, but periodontal disease mainly develops due to the enzymes secreted during the metabolism of Gram-negative anaerobic bacteria [9,10]. Although most of the periodontal disease-causing bacteria in dogs are different from those in humans, some of the species are common. Additionally, several periodontopathic species can be transmitted between humans and dogs [11]. Of these, three bacteria, Treponema denticola, Tannerella forsythia, and Porphyromonas gingivallis, are mainly associated with periodontitis in both dogs and humans; they are usually referred to as the red complex bacteria (RCB) [12,13].
Periodontitis is the most prevalent disease in dogs, affecting approximately 80% of dogs aged 4 years [14]. However, since the oral care in dogs is more easily neglected than in humans, gingivitis can rapidly progress to periodontitis without proper prevention and therapy.
Traditional clinical methods, such as pocket probing depth and bleeding on probing, are widely used in veterinary dentistry for assessing periodontal disease [15]. However, they do not provide sufficient information regarding the presence and absence of bacteria. Therefore, there is an increased interest in developing diagnostic methods for the prevention and treatment of periodontitis in dogs, which would be equally important for the oral health in humans.
The microbial-enzymatic N-benzoyl-DL-arginine-2-naphthylamide (BANA) has a unique ability to hydrolyze the trypsin substrate, and it can be used to detect the presence of the three putative pathogens in subgingival plaque samples known as RCB [3]. Furthermore, a previous study reported a strong relationship between a BANA-positive result and a high level of plaque spirochetes [16,17]. Accordingly, a BANA-based periodontitis diagnostic test kit was developed for use in humans [3,18]. However, especially in Korea, there are not many diagnostic test kits for assessing periodontitis in dogs using BANA yet.
MiriChekTM Oral Bacter (O-Bacter; Mirimedix Incorporation, Chuncheon-si, Gwangwon-do, Korea) was developed as a rapid diagnostic BANA test kit, but formal clinical research have not yet been conducted.
In this clinical and in vivo study, we aimed to determine the usefulness of the O-Bacter as compared to the traditional clinical methods in the periodontal assessment of dogs with and without periodontitis.
We hypothesized that the O-Bacter test kit can detect the presence of BANA in plaque and help in diagnosing periodontitis in dogs, thereby achieving better prevention and treatment.
Methods
This study was conducted after explaining the diagnostic test kit method to the owners of 61 dogs visiting the veterinary clinic at Pet in zoo animal medical center, Uijeongbu-si, Gyeonggi Province and obtaining their consent. Clinical diagnosis of periodontitis was determined by two veterinarians based on probing depth, bleeding on gentle probing, and visual inspection. Briefly, healthy tooth and gums (stage 0), the gingival or gum tissue is a pink or coral color. The tissue is firm and resilient, and has minimal pocket depth. Gingivitis (stage 1), the gingival or gum tissue will be inflamed at the neck of the teeth, as opposed to the pinkish color, and there will be pocket depth and gingival bleeding on probing. And, was observed variable amounts of plaque and mildly reddened gums. Early periodontitis (stage 2), inflammation of periodontal ligaments and minor loss of attachment and pocket development. But, no tooth mobility at this stage. And, was observed larger amounts of plaque and bleeding gums. Moderate periodontitis (stage 3), moderate loss of attachment and/or moderate to deep pocket formation, and slight tooth mobility. Advanced periodontitis (stage 4), advanced breakdown of supporting periodontal tissue and severe pocket depth or significant gingival recession, and severe loss of tooth attachment. And, was observed dead tissue with blood and pus [19].
The experimental protocols used in this study were approved by the internal animal care and use ethics committee of the Seojeong university (IACUC Approval Number: SJ2021-03), Korea.
1. Animals
In this study, the breed, age, sex, weight, and presence of other diseases were recorded for 61 dogs. Of these, dogs with and without periodontitis (n=30 and n=31, respectively) were identified using the plaque index, bleeding index, and periodontal pocket depth scores of at least four sites as the dental clinical parameters.
2. Periodontitis diagnostic kit
The O-Bacter test kit was provided by Mirimedix Incorporation <Fig. 1>. Specimen collection and procedures were performed according to the manufacturer’s instructions.
3. Study methods
1) Specimen collection and procedure
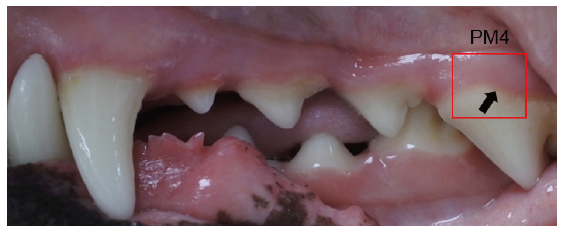
The supragingival plaque was collected from the upper left premolar using a cotton swab <Fig. 2>. The collected plaque sample was placed in a tube containing the specimen reaction reagent. The solution was stirred well with the swab to extract the plaque from the swab thoroughly. After removing the swab, the cap of the tube was closed and mixed well using a vortex mixer for 15 seconds or shaken by hand 10 times. The tube was placed on a heat block at 50℃ for 10 minutes or at a room temperature of 20–28℃ for 20 minutes. After the reagent reaction, the tube was turned upside down twice and mixed. The cap was then opened and the sample solution was drawn using a disposable dropper, and two drops were transferred into the test device inlet. The results were checked after 5 minutes. The result was considered positive if the test line changed color (dark blue) and negative if both the test line and control line were orange.
2) Analysis of the test line color intensity
For quantitative comparison, the color intensities were measured using scanned images with Image J software (National Institutes of Health, https://imagej.nih.gov/ij/download.html) and compared with the untreated control line. The color intensity was normalized to the intensity of the untreated control (first test line).
4. Statistical analysis
The data were statistically analyzed using the student’s t-test for comparison between the two groups and one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons using graph pad prism 4 for windows statistical software package (Graph Pad Software Inc., La Jolla, CA, USA). p<0.05 were considered statistically significant.
Results
1. Characteristics of the study animals
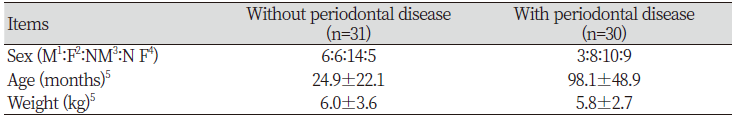
The sex, average age, and average weight of the animals are presented in <Table 1>, and the individual characteristics are shown in detail in <Table 2,3>. The sex distribution among the dogs without periodontitis (n=31) was as follows: 6 males (19.4%), 6 females (19.4%), 14 neutered males (45.2%), and 5 neutered females (16.1%). The sex distribution among the dogs with periodontitis (n=30) was as follows: 3 males (10.0%), 8 females (27.7%), 10 neutered males (33.3%), and 9 neutered females (30.0%). The ratio of neutering was high (62.3%) among the animals included in this study.
The average age of the 61 dogs was 61 months, with 33 dogs aged between 1 month and 48 months (54.1%) and 12 dogs aged over 120 months (19.7%). The oldest dog was 180 months old. The average age of the dogs with periodontitis was significantly higher than that of the dogs without periodontitis (98.1±48.9 months vs. 24.9±22.1 months; p<0.001).
The average weight of the dogs in this study was 5.8±3.1 kg. The average weight of the dogs with and without periodontitis was 5.8±2.7 kg and 6.0±3.6 kg, respectively, without a significant difference (p>0.05).
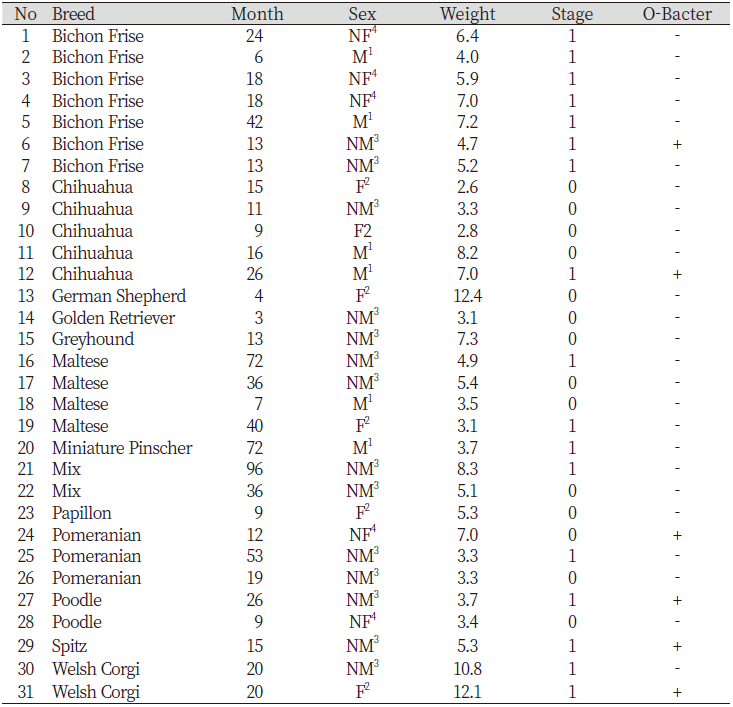
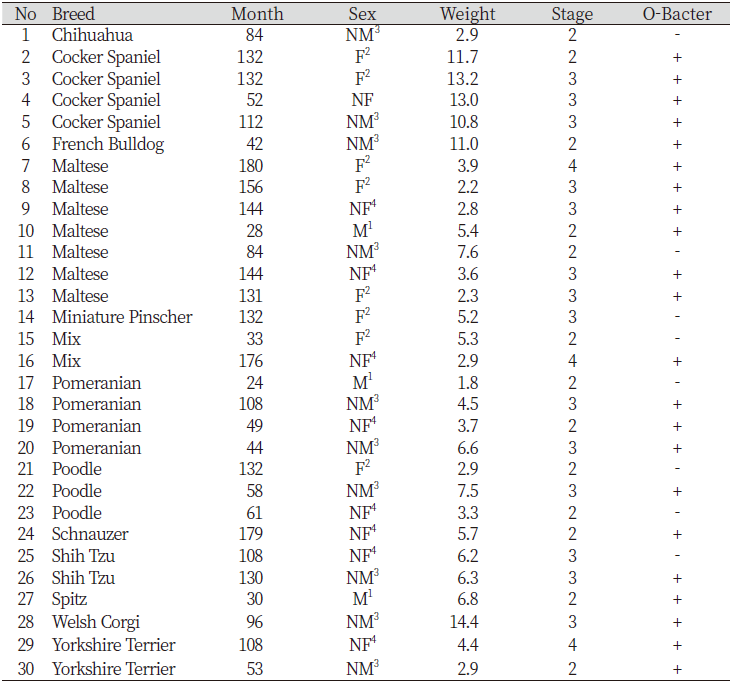
The distribution of the breeds among the 61 dogs was as follows: Maltese, 11 (18.0%); Bichon frise, 7 (11.5%); Pomeranian, 7 (11.5%); Chihuahua, 6 (9.8%); Poodle, 5 (8.2%); Mixed, 4 (6.6%); Cocker spaniel, 4 (6.6%); Welsh corgi, 3 (4.9%); Miniature pinscher, 2 (3.3%); Yorkshire terrier, 2 (3.3%); Shih tzu, 2 (3.3%); Spitz, 2 (3.3%); German shepherd, 1 (1.6%); Golden retriever, 1 (1.6%); Greyhound, 1 (1.6%); Papillon, 1 (1.6%); French bulldog, 1 (1.6%); and Schnauzer, 1 (1.6%).
The 31 dogs without periodontitis included 7 Bichon frises (21.7%), 5 Chihuahuas (15.5%), 1 German shepherd (3.1%), 1 Golden retriever (3.1%), 1 Greyhound (3.1%), 4 Malteses (12.4%), 1 Miniature pinscher (3.1%), 2 Mixed (6.2%), 1 Papillon (3.1%), 3 Pomeranians (9.3%), 2 Poodles (6.2%), 1 Spitz (3.1%), and 2 Welsh corgis (6.2%). The 30 dogs with periodontitis included 1 Chihuahua (3.0%), 4 Cocker spaniels (12.0%), 1 French bulldog (3.0%), 7 Malteses (21.0%), 1 Miniature pinscher (3.0%), 2 Mixed (6.0%), 4 Pomeranians (12.0%), 3 Poodles (9.0%), 1 Schnauzer (3.0%), 2 Shih tzus (6.0%), 1 Spitz (3.0%), 1 Welsh corgi (3.0%), and 2 Yorkshire terriers (6.0%).
2. Comparison of clinical periodontitis stage with the O-Bacter test kit score results
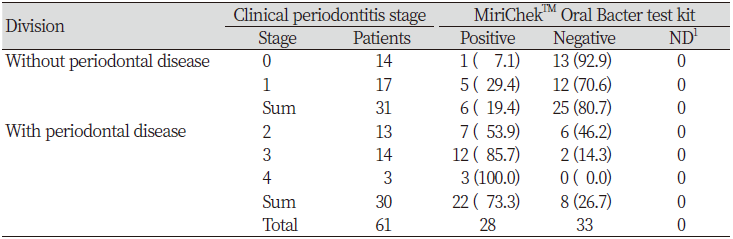
The clinical examinations of the 61 dogs revealed periodontitis stage 0 in 14 dogs, stage 1 in 17 dogs, stage 2 in 13 dogs, stage 3 in 14 dogs, and stage 4 in 3 dogs <Table 4>. The individual test scores are shown in <Table 2,3>.
Table 1. Characteristics of the dogs in the study
|
|
1Male; 2Female; 3Neutered male; 4Neutered female; 5Mean±SD *by student’s t-test |
Table 2. Detailed characteristics of the dogs without periodontitis
|
|
1Male; 2Female; 3Neutered male; 4Neutered female |
Table 3. Detailed characteristics of the dogs with periodontitis
|
|
1Male; 2Female; 3Neutered male; 4Neutered female |
Table 4. Comparison of the MiriChekTM Oral Bacter test kit results and the clinical periodontitis
stage in dogs Unit : N(%)
|
|
1No detection |
The O-Bacter test kit result was positive in 1 dog (7.1%) and negative in 13 dogs (92.9%) of the 14 periodontitis stage 0 dogs, positive in 5 (29.4%) and negative in 12 (70.6%) of the 17 stage 1 dogs, positive in 7 (53.9%) and negative in 6 (46.2%) of the 13 stage 2 dogs, positive in 12 (85.7%) and negative in 2 (14.3%) of the 14 stage 3 dogs, and positive in all 3 (100.0%) stage 4 dogs. Overall, the average positive and negative sensitivity rates of the O-Bacter test kit were 73.3% and 80.7%, respectively.
3. Interpretation of O-Bacter test kit results
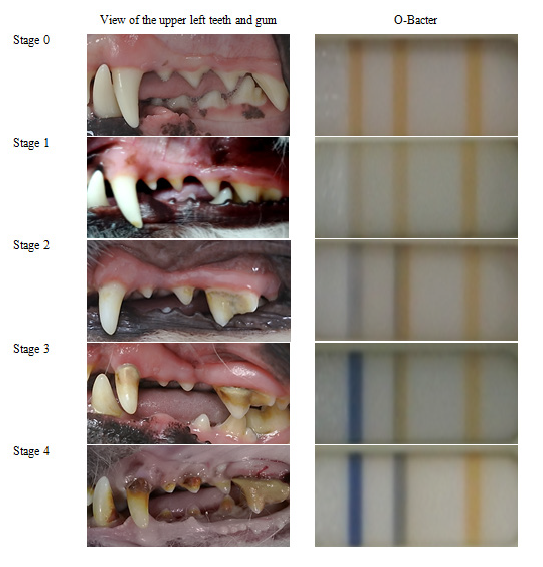
A comparison of the clinically determined periodontitis stage and the O-Bacter test results is presented in <Fig. 3>. Periodontitis stage 0 was characterized by bright red gums and clean teeth, with no change in the first test line and second test line. Periodontitis stage 1 was characterized by slightly red gums and discolored teeth, and the color of the first test line was faded. Swollen gums and plaque were observed in periodontitis stage 2, and the color of the first test line changed to light blue. Swollen gums, plaque, yellowish teeth, slight gingival recession, and change in the color of the first test line to complete blue were observed in periodontitis stage 3. Periodontitis stage 4 was characterized by bleeding gums, plaque, yellowish teeth, and exposure of tooth roots; additionally, the color of the first test line changed to complete blue, while that of the second test line changed to light blue.
4. Analysis of O-Bacter test kit control line color intensity
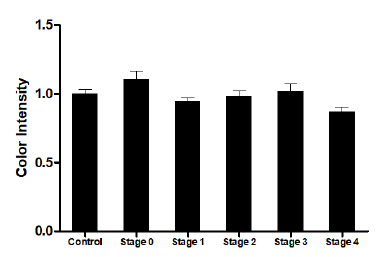
The color intensity changes in the first test line of the O-Bacter kit are shown in <Fig. 4>. Periodontitis stage 0 showed 1.1±0.2; stage 1, 0.9±0.1; stage 2, 1.0±0.2; stage 3, 1.0±0.2; and stage 4, 0.9±0.1 compared to control, with no significant difference (p>0.05).
Discussion
Dogs of all breeds are at a risk of developing periodontal disease, which is caused by infection and inflammation due to the toxic enzymes secreted from the bacteria in the plaque [7,20]. Periodontal disease is divided into the following four stages based on the severity of the clinical manifestations: stage 0 (normal), stage 1 (gingivitis), stage 2 (early periodontitis), stage 3 (moderate periodontitis), and stage 4 (advanced periodontitis) [21]. Most veterinarians still use clinical experience and probes to determine the periodontal disease stage in dogs with clinical manifestations. However, these clinical assessment methods may provide insufficient information for the prevention and treatment of periodontal disease in dogs.
Currently, research and development of periodontitis diagnostic test kits for human plaque has seen a growing interest in the use of BANA [3,18]. However, we have limited knowledge about its application in dogs. O-Bacter is a BANA-based periodontitis diagnostic test kit for dogs. The objective of this study was to evaluate the usefulness of O-Bacter as compared to the traditional clinical methods of periodontal disease assessment in dogs.
In this study, periodontitis was found to be more common in the older dogs than in the younger ones, but there were no clinical manifestations observed due to differences in weight <Table 1>. Periodontal disease is a common disease affecting adult dogs, and it has been reported in 80% of dogs aged 4 years, and the incidence increases with age [14]. In this study, periodontal disease showed an association with the dog breed, but not body weight; dog breeds with brachycephaly and overcrowding of teeth are especially vulnerable [22]. Unfortunately, our data comparisons between groups did not reflect age as a control variable, and could not confirm the breed-related differences. As such, the above limited of data requires future study. These results suggest that as age increases in dogs, dental care becomes more important.
The positive and negative sensitivity rates of the O-Bacter test kit were 73.3% and 80.7%, respectively, when compared with the clinically determined periodontitis stages <Table 4>. The sensitivity of the O-Bacter test kit was slightly lower than that of the in vivo BANA-ZymeTM test kit for humans as reported by Dhalla et al. [3]. BANA has shown potential as a periodontal disease marker by assessing the probing depth and the proportion of spirochetes in plaque [16,17]. Moreover, a previous study showed that BANA-ZymeTM was useful in detecting the RCB of periodontal pathogens [3]. However, the result for periodontitis stage 2 (sensitivity: 53.9% positive and 46.2% negative) was unclear. This low sensitivity in determining periodontitis stage 2 might be related to the low intensity of RCB; hence, there is a need for measures to compensate for the low sensitivity.
The O-Bacter test kit determines the results based on the color change of the pad test line. Our results showed that there was no color change in periodontitis clinical stage 0, the color faded in periodontitis clinical stage 1, the color changed to light blue in periodontitis clinical stage 2, the color changed to complete blue in periodontitis clinical stage 3, and the color of the first line changed to complete blue and that of the second line changed to light blue in periodontitis clinical stage 4 <Fig. 3>. The readability and ability to detect the degree of periodontitis of the O-Bacter were similar to those of BANA-ZymeTM [3]. Furthermore, we quantified the change in the color intensity of the first line of the pad using Image J <Fig. 4>. Based on these results, we identified the potential for improving the method of diagnosing the periodontitis stage using assist devices that can measure the intensity of color change in the future. Overall, these results are useful for the diagnosis of periodontitis in dogs and may pave the way for further research for using the O-Bacter test kit in humans.
Conclusions
This study aimed to provide alternative methods for the diagnosis of periodontal diseases in dogs using the O-Bacter test kit.
1. The periodontitis was more common in the older dogs than in the younger ones.
2. The average positive and negative sensitivity rates of the O-Bacter test kit were 73.3% and 80.7%, respectively, with high readability.
3. We identified the potential for improvement in the stepwise diagnosis method for periodontitis based on the color intensity.
4. In conclusion, the O-Bacter test kit can assist in the diagnosis of periodontal disease in dogs.
Authorship
Conceptualization: H Choi, IS Hwang, YE Jeong; Data collection: IS Hwang, WD Park; Formal analysis: MS Kim, H Choi; Writing - original draft: H Choi, Writing - review & editing: H Choi, YJ Kim, JH Roh, IS Hwang, WD Park, MS Kim, YE Jeong
초록
연구목적: 본 연구는 개 치주염 평가에서 MiriChekTM Oral Bacter test kit의 임상적 유용성을 판단하기 위해 실시하였다. 연구방법: 개 61두를 공시하여 품종, 연령(개월), 성별, 체중, 질병 유무, 임상적 치주염 단계를 수의 임상 진단 후 상악 왼쪽 전구치의 치태를 채취하여 MiriChekTM Oral Bacter test kit를 이용하여 진단검사를 수행하였다. 연구결과: 대상 개 61두 중 건강한 개는 31두, 치주염 개는 30두였고, 치주염은 젊은 개보다 나이 든 개에게서 더 흔하게 나타났다. MiriChekTM Oral Bacter test kit의 양성 평균 73.3%, 음성 평균 80.7%의 민감도를 보였고, 치주염의 심각도와 높은 상관관계를 확인하였으며, 그 결과 MiriChekTM Oral Bacter test kit는 개 치주염 평가에 유용한 진단검사 방법임을 확인하였다. 결론: MiriChekTM Oral Bacter test kit와 같은 새로운 진단 보조 기구의 활용은 개에게서 발생하는 치주질환 진단의 신뢰성과 정확성을 높일 것으로 판단된다.색인: 반려동물, 신속진단키트, 적색 박테리아 집합, 치주질환, N-benzoyl-DL-arginine-2-naphthylamide