Introduction
The success of a root canal treatment relies on effectively removing these bacteria and sealing the canal properly to avoid recontamination from the products. Therefore, many clinical practices apply preventative treatments by using various root canal medications. Although many new medication pastes have been developed and released commercially, calcium hydroxide paste has been the most widely used product for more than a century. Calcium hydroxide is known to exert various antibacterial properties[1,2], induce the mineralization of hard tissue[3,4] promote lysis of necrotic tissues and bacterial endotoxin in vitro and in vivo[5], and it has been used generally in clinic.
Hard tissues in the human body, including the tooth, mainly consist of hydroxyapatite (HA) and this has been used as synthesis bone graft materials until now. To enhance the bone formation, some researchers modified the HA in many ways. In particular, since silicon is an essential ion for hard tissue formation, silicon-substituted hydroxyapatite (Si-HA) has been developed for use as bone graft materials. Previous studies have shown the osteogenic activity and osteoinductibility of Si-HA[6]. Torres et al.[7] and Stanić et al.[8] demonstrated the antibacterial properties of HA and Si-HA. So we added Si-HA in calcium hydroxide paste to enhance its hard tissue formation property. The synthesis HA was made from natural coral and approximately 1% of silicon was substituted in HA.
For the positive effect on the bone, the percentage of calcium hydroxide was reduced by adding the Si-HA in the product. Despite of the lower percentage calcium hydroxide, we were wonder that the antibacterial property was remained same as before. So, in the previous study, we focused on the antibacterial property of the calcium hydroxide paste containing Si-HA with comparing the commercial product.
Infection of the root canal can be caused by various microorganisms, including gram-negative bacteria that release lipopolysaccharide (LPS)[9,10]. To mimic infected root canal, LPS-treated cell model has been used in vitro. In a previous study, the antibacterial effect of calcium hydroxide paste was conducted in the LPS-treated RAW 264.7 cells and they reported that it showed significant results in antibacterial[11].
Activation of macrophages, among many immune cells, plays an important role in the inflammatory process by the production of various cytokines and chemokines. LPS, a main component of endotoxin, induces the activation of macrophages, which then increases the expression of inducible nitric oxide synthase (iNOS), causing the overproduction of nitric oxide (NO)[12]. NO plays an important role in inflammatory immune responses and in the host defense against various pathogens such as bacteria and viruses[13]. However, high amounts of NO have cytotoxicity and induce tissue damage associated with acute and chronic inflammations. The RAW 264.7 macrophage cell line has been shown to have an enhanced inflammatory response to endotoxin[14-16]. Thus, the aim of this study was that we evaluated the effect of the newly developed calcium hydroxide paste containing Si-HA on anti-inflammatory activities in LPS-stimulated RAW 264.7 macrophages.
Materials and Methods
1. Material
To make calcium hydroxide paste, we blended Si-HA (6%), calcium hydroxide paste (20%) with polyethylene glycol (Sigma-Aldrich, St. Louis, MO, USA), polypropylene (Sigma-Aldrich), distilled water and radiopaque agent. The information of the test group and product control was shown in <Table 1>.
2. Preparation of silicon substituted hydroxyapatite (Si-HA)
The natural Goniopora coral (CaCO3, Indonesia) of interconnected pore (300-500 μm) was obtained. In briefly, the corals were bleached in 12-13% sodium hypochlorite solution for 24 h and put under sonication to remove debris. The coral blocks were placed in a hydrothermal reactor (iNexus, Seoul, Korea) and heated for 24 h at 200℃. The calcium carbonate was converted into HA by immersing in tetraortho silicate solution (Sigma-Aldrich) and reacted at 60℃ to synthesize silicon-substituted HA for 48 h. About 1%(wt) silicon was changed into the HA structure, and its silicon content was analyzed by X-ray fluorescence (XRF, Shimadzu, Kyoto, Japan) and inductively coupled plasma (ICP; NexION300, PerkinElmer, Waltham, MA, USA). For the phase analysis of HA, X-ray diffraction (XRD; Ultima IV, Rigaku, Germany) was taken as in a previous study[17,18].
3. Measurement of pH and calcium ions
The pH of the solutions was measured using a pH meter (Orion4 star; Thermo Fisher Scientific Inc., Waltham, MA, USA). For quantification of the calcium ions in solution, we used the QuantiChrome calcium assay kit (BioAssay Systems, Hayward, CA, USA). Briefly, after mixing the two regents at 100 ㎕ each, 5 ㎕ of the extraction was added to a 96-well microplate and incubated with shaking for 5 min. Absorbance was measured at 612 nm using a microplate reader (Epoch, Winooski, VT, USA). The optical density values were calculated by the standard curve.
4. Cell culture
The murine RAW 264.7 macrophages were cultured in Dulbecco’s modified Eagle’s medium (DMEM) including 2 mM L-glutamine, 100 U/㎖ penicillin, 100 ㎕/㎖ streptomycin, and 10% heat-inactivated fetal bovine serum in a humidified atmosphere of 5% CO2 at 37℃.
5. MTT assay
RAW 264.7 cells were plated at a density of 5 × 103 cells/well in a 96-well plate and left overnight to adhere at 37℃, in a 5% CO2 incubator. The attached cells were treated with various concentrations of Calcipex II or TRC paste for 24 h. Viable cells were detected using a MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution. The absorbance was measured at 570 nm in a Benchmark microplate reader (Bio-Rad, Hercules, CA, USA).
6. Measurement of NO production
The nitrite, as an indicator of NO production, was measured by the Griess reaction method[19]. RAW 264.7 cells were plated at 5 × 104 cells/well in a 96-well plate. Cells were washed with PBS and incubated with 1 ㎍/㎖ LPS in the presence or absence of Calcipex II or TRC paste. After 24 h incubation, 100 ㎕ of the culture supernatant was blended with 100 ㎕ of Griess reagent [sulfanilamide in phosphoric acid and N-(1-naphtyl) ethylenediamine] in a new 96-well plate and then reacted at room temperature for 10 min. The absorbance at 540 nm was measured in a microplate reader (Bio-Rad). The amount of nitrite was calculated according to the sodium nitrite serial dilution as a standard curve.
7. Western blot analysis
RAW 264.7 cells were treated with 1 ㎍/㎖ LPS in the presence or absence of Calcipex II or TRC paste for 24 h. Cell lysis was performed with RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) containing 1 mM PMSF and protease inhibitor cocktail tablets (Roche, Mannheim, Germany) on ice for 1 h. Cell lysates were centrifuged and then the supernatants were collected. Protein content was quantitated using BCA protein assay reagents (Pierce, Rockford, IL, USA). Proteins were separated by 7.5% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane (Millipore, Danvers, MA, USA). The membrane was blocked with 5% skim milk in PBS containing 0.1% Tween-20 for 1 h at room temperature and incubated with primary antibodies against iNOS and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight 4℃. The blots were incubated with a 1:2000 dilution of the respective horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology) for 2 h at room temperature, and the target proteins were detected using the Amersham ECL western blotting detection kit (GE Healthcare, Piscataway, NJ, USA).
8. Statistical analysis
Data are expressed as the mean ± standard deviation (SD) of three independent experiments and analyzed via one-way analysis of variance with multiple comparisons using SPSS (SPSS Inc., Chicago, IL, USA). p-values of less than 0.05 were considered statistically significant.
Results
The result of pH and calcium ion quantities in the extraction solutions of Calcipex II and TRC paste were in <Table 2>. The pH of Calcipex II and TRC paste was about 12.7 and 12.5, respectively. The calcium ion concentrations were 29.8 ± 0.43 mg/㎗ and 31.4 ± 0.58 mg/㎗ in Calcipex II and TRC paste, respectively. The cell culture medium used in this study had a calcium ion concentration.
|
Table 2. The pH and calcium ion concentration of test materials |
||
|
Name |
pH(n=3) |
Ca2+(mg/dL)(Mean ± SD) |
|
Culture media (DMEM) |
≒ 7.4 |
6.6 ± 0.30 |
|
Calcipex II |
≒ 12.7 |
29.8 ± 0.43 |
|
TRC paste |
≒ 12.5 |
31.4 ± 0.58 |
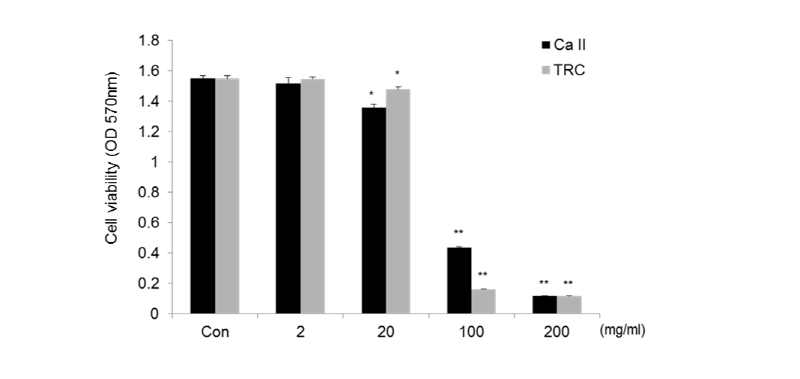
To investigate the cytotoxicity of Calcipex II or TRC paste, RAW 264.7 cells were treated with various concentrations of material extraction solution for 24 h, and then cell viability was measured by an MTT assay. As shown in <Fig. 1>, the extraction solution 200 mg/㎖ without dilution of the two materials showed substantial cytotoxicity. The viability of the cells was 98% and 88% with 2 mg/㎖ and 20 mg/㎖ of Calcipex II, compared with untreated cells (Con). TRC paste at 2 mg/㎖ and 20 mg/㎖ resulted in slightly higher viability of 100% and 96% than Calcipex II. Therefore, neither Calcipex II nor TRC paste was cytotoxic at doses below 20 mg/㎖ in RAW 264.7 cells.
The two materials were evaluated for the inhibitory effect of NO production in RAW 264.7 cells stimulated by LPS. The amount of NO was measured as nitrite concentration in the conditioned media. As shown in <Fig. 2>, LPS at a concentration of 1 ㎍/㎖ induced about a 4-fold increase in nitrite formation from the basal level of the control. However, treatment of Calcipex II or TRC paste dose-dependently inhibited the formation of NO in LPS-stimulated RAW 267.4 cells. The IC50 values of Calcipex II and TRC paste were 17.6 mg/㎖ and 13.5 mg/㎖, respectively. In particular, TRC paste at 20 mg/㎖ exhibited a significant decrease of nitrite to 14.4 μM, representing an inhibition rate of 65%.
To confirm whether the inhibitory effect of the materials on NO production was attributable to the regulation of iNOS expression, western blotting was conducted on the cell lysates for iNOS measurements. <Fig. 3> shows the normalized intensity values of iNOS to β-actin following treatment relative to the untreated control. The expression of iNOS protein was decreased by Calcipex II or TRC paste in a dose-dependent manner. In particular, TRC paste at 2 mg/㎖ and 20 mg/㎖ significantly inhibited the levels of iNOS protein by 71% and 92%, respectively.
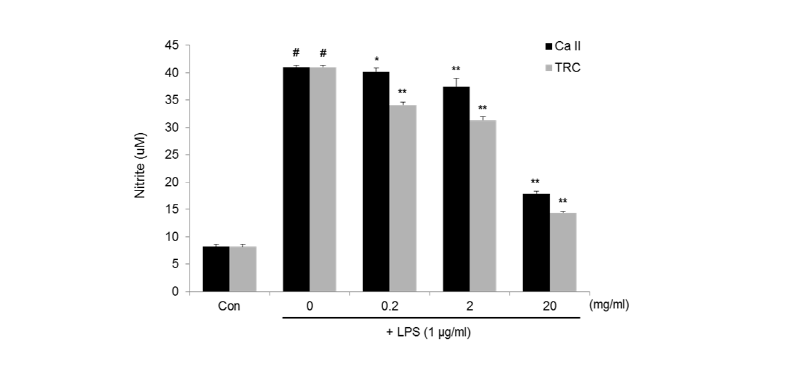
Fig. 2. NO production in LPS-stimulated RAW 264.7 cells treated with various concentrations of Calcipex II or TRC paste for 24 h. Amount of NO was measured as nitritis accumulation in the conditioned medium, and data are expressed as mean ± SD. #p < 0.01 vs Con, *p < 0.05, **p < 0.01 vs LPS alone group (0 mg/ml).
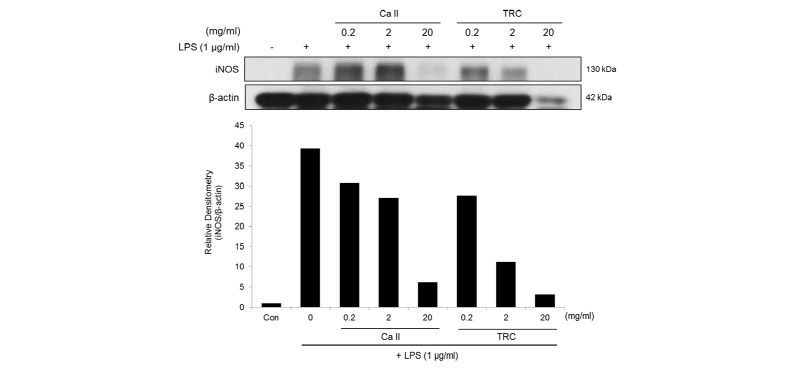
Fig. 3. NO production in LPS-stimulated RAW 264.7 cells treated with various concentrations of Calcipex II or TRC paste for 24 h. Amount of NO was measured as nitritis accumulation in the conditioned medium, and data are expressed as mean ± SD. #p < 0.01 vs Con, *p < 0.05, **p < 0.01 vs LPS alone group (0 mg/ml).
Discussion
Calcium hydroxide paste has long been used as a root canal antibacterial and a hard tissue reparative treatment. Among the various characteristics of calcium hydroxide paste, many researchers have focused on its antibacterial activity, dentin formation, and anti-inflammation properties[20,21].
In general, calcium hydroxide pastes can be classified into water- or oil-based materials. Compared to the oil-based paste, water-based calcium hydroxide paste has been used for mature endodontic treatment owing to property of easy removal. However, some weaknesses or risks associated with water-based calcium hydroxide paste have been reported in clinical cases. Kim et al.[22] reported clinical hazardous risks caused by an overfilled material over the root apex, such as alveolar bone resorption and necrosis[23]. Therefore, we modified and focused on a material that includes similar ingredients to those naturally found in alveolar bone.
The synthetic hydroxyapatite (HA) showed a similarity with natural bone in bone mineral and it is desirable for bone growth and many modification have been tried. Silicon is one of key element of hard tissue formation, and has been detected in various connective tissues. To take its advantages, silicon substituted HA was used in bone graft materials[18].
To confirm the character of modified calcium hydroxide paste, we used premarket product and our experiment group. The Calcipex II, which was used as the premarket product for this study, is a water-based calcium hydroxide paste for a root canal filling. The experimental modified calcium hydroxide paste by the addition of Si-HA, TRC paste, was developed to enhance the healing effects on bone tissue. The mainly different ingredient between the two materials is Si-HA, which showed an essential role during the mineralization and also reported as a basic factor in collagen[18]. Sutha et al.[24] reported the biocorrosion and antibacterial activity of Si-HA. Thian et al.[25] confirmed the effects of Si-HA on differentiation of osteoblasts, and Patel et al.[26] got an in vivo result that bioactivity of HA was improved by adding silicate ions into the HA. These previous researches suggested that Si-HA might have potential as a supplementary material for a bone graft and osteoporosis treatment. We investigated the anti-inflammatory property of a calcium hydroxide paste containing Si-HA to determine its applicability in root canal treatment.
First, we evaluated the pH value and quantity of calcium ions in extraction solutions of Calcipex II and TRC paste. The pH range was similar in the extraction solution of the two materials, but the calcium ions concentration was higher in the TRC extraction. The hydroxyl and calcium ions are dissociated out of the calcium hydroxide paste in the aqueous phase. Although these two ions are known to induce cell responses, including toxicity, there is currently no clear understanding of the mechanisms underlying the cellular effects of calcium hydroxide paste[21,27]. In a previous study, the cytotoxicity was confirmed using original and pH-adjusted extraction solutions of calcium hydroxide paste, which showed that the pH did not affect cell proliferation[3]. However, since a high calcium ion concentration disrupts the calcium balance inside and outside of the cellular membrane, it is possible that apoptosis could be occurred.
We conducted MTT assay to determine the non-cytotoxic concentration of the extraction solutions of the two materials. The extraction solution of TRC paste below 20 mg/㎖ showed a survival rate of 95% or more. Thus, the addition of Si-HA to the calcium hydroxide paste does not affect the safety of the existing product, and instead showed lower cytotoxicity than Calcipex II.
Subsequent experiments were carried out at the non-toxic concentration (below 20 mg/㎖) of the two materials, and their anti-inflammatory activity was investigated in LPS-stimulated RAW 264.7 cells. NO plays an important role in the regulation of immunological systems as a host defense effector, and particularly mediates the activation of macrophages under the inflammatory response[13,28]. In macrophages, iNOS is overexpressed by endotoxins or cytokines during inflammation and produces a large amount of NO. Therefore, the suppressive effect of NO and iNOS is an essential property of an anti-inflammatory agent. In this study, we measured nitrite, the final product of NO oxidation, and iNOS at the protein level. The results showed that Calcipex II and TRC paste dose-dependently decreased NO production and iNOS expression induced by LPS in RAW 264.7 cells. The TRC paste showed higher attrition rates than Calcipex II at all concentrations tested in assay for NO and iNOS. Our results demonstrate that calcium hydroxide paste significantly inhibits inflammatory activity in LPS-stimulated macrophages, and the effect is enhanced when Si-HA is added to the paste. In western blotting analysis, however, level of β-actin protein were decreased in both test paste at 20 mg/ml. Repeated experiments showed the same tendency. Although further studies are needed, this data suggests that a high concentration of calcium hydroxide paste may affect the cytoskeletal structure.
Despite the fact that calcium hydroxide paste shows positive effects in endodontic treatment, there has been no clear evidence pointing to the underlying mechanism of these beneficial effects. It has been suggested that calcium hydroxide paste as a root filling material could stimulate the periapical tissue to maintain health or promote healing. In addition, the release of ions could increase the pH, which would encourage repair and active calcification[4,5,20]. Although these mechanisms could explain the healing effects of calcium hydroxide paste, its mechanism of anti-inflammatory activity remained unclear. The present results showed that the newly developed TRC paste showed a clear anti-inflammatory effect in macrophages stimulated by LPS. In conclusion, the newly developed TRC paste significantly decreased the production of NO and iNOS compared to the control group. To confirm the other properties of TRC paste containing Si-HA, further experimental such as bone mineralization and clinical studies are recommended.
Conclusions
The anti-inflammation effect of modified Calcium hydroxide, TRC paste containing silicon-substituted hydroxyapatite (Si-HA) were investigated using MTT assay, nitric oxide production and inducible nitric oxide synthase expression in lipopolysaccharide-treated RAW 264.7 cells. From the results, the Si-HA calcium hydroxide paste has improved anti-inflammatory property compare to control product without Si-HA. To confirm its mechanism and other application in clinics, further studies such as mineralization tests are needed before clinical recommendations can be proposed.