Introduction
The incidences of thyroid cancer have been increasing over the last three decades and accounts for approximately 1% in the prevalence of all cancers [1-3]. A recent report showed the world-wide mortality rates of thyroid cancer were 1.9 per 100,000 in men and 6.1 per 100,000 in women [4]. Among the three types of thyroid cancer, indicating papillary, follicular, and medullary thyroid cancers, a papillary thyroid cancer is the most prevalent representing 85% of all thyroid cancer cases [5,6]. A radiation exposure is a widely known risk factor for thyroid cancer. Previous studies consistently showed the significant correlation between high-dose radiation exposure and thyroid cancer among atomic bomb survivors in 1945 and residents near the Chernobyl nuclear power plant accident in 1986 [7-9].
A medical use of X-ray in head and neck area for diagnostic and therapeutic purposes is a prominent source of ionizing radiation on the thyroid gland. There is a growing concern on radiation-induced thyroid cancer due to frequent exposures of diagnostic radiation during routine medical care. For example, over one third of diagnostic computed tomography scans are performed in the region of the head and neck of patients [10]. Generally, levels of cancer risk from radiation exposure differ by age of people. Specially, children tends to develop radiation-induced cancer more easily compared to adults because mutagenic effect of radiation is larger on growing tissues. Therefore, previous studies have examined the effects of medical radiation on thyroid cancer among different radiation types and age groups [11-13].
Dental radiation exposure is also of potential concern on thyroid cancer risk because of the anatomic position of thyroid that is adjacent to teeth and mouth. Dental radiographs are routinely used at dental clinics to diagnose diseases, to set up treatment plans, and to check for treatment progress [14]. Although the exposure level by dental radiation is less than that of medical imaging, previous studies showed that dental X-ray exposure can lead to the potential risk of cancer [15-17]. Therefore, the intrinsic risk from the small amount of radiation exposure from dental X-ray cannot be disregarded.
Despite the potential risk of dental radiation for thyroid cancer, only a few recent case-control studies have examined the association between dental radiation exposure and thyroid cancer incidence [18-21], showing inconsistent results [22,23]. Therefore, performing a meta-analysis summarizing reported inconsistent effects as well as more elaborated studies to provide further evidences. Although a recent meta-analysis on medical radiation exposure and thyroid cancer suggested a possible association of dental X-ray exposure with increasing thyroid cancer [24], specific study characteristics that affect the association between dental X-ray exposure and thyroid cancer risks have not been examined. We conducted a meta-analysis in order to systematically assess the inconsistent results of previous research and to summarize the effect sizes of association between exposure to dental radiation and the risk of thyroid cancer, reported from the previous case-control studies. In addition, we compared the results by subgroups with various study characteristics.
Methods
1. Study inclusion and exclusion
The current meta-analysis was executed in accordance with the guidelines for Meta-analyses of Observational Studies in Epidemiology (MOOSE) [25]. The systematic review process was conducted using the patient, intervention, comparator, and outcomes (PICO) framework. A study was included if the following inclusion criteria were satisfied: the study (ⅰ) examined dental X-ray exposure as an independent variable, (ⅱ) used odds ratios (OR) of thyroid cancer with a corresponding 95% CI or relative risk (RR) with a corresponding 95% CI, (ⅲ) it was a human study, and (ⅳ) the full text of the study was available. The exclusion criteria were as follows: (ⅰ) a radiation dose assessment study and (ⅱ) a radiation safety management study were excluded because they did not assess radiation exposure nor cancer occurrence of patients. In addition, (ⅲ) a review article, or (ⅳ) a case report was excluded.
2. Search strategy
To identify relevant studies on exposure to dental X-rays and the effects on thyroid cancer risk, two authors (SYH & ESC) independently reviewed titles and abstracts according to the inclusion and exclusion criteria. When it was difficult to assess a study by reading only the abstracts, the full text of the study was reviewed. Any discrepancy between reviewers was resolved through discussion. All related studies published in English before September 2018 were searched. To do this, a systematic literature search of the PubMed and EMBASE databases was performed using the following search terms: (dental X-rays OR dental radiography) AND (thyroid cancer OR thyroid). Furthermore, the reference lists of the selected studies were reviewed.
3. Data extraction
Two authors independently collected the following basic information: first author’s name, year of publication, number of participants (case/controls), age, sex, outcome, OR with 95% CI, and variables adjusted for the analysis.
4. Quality assessment
The Newcastle-Ottawa scale (NOS) was used to assess the quality of the case-control studies [26]. Each study was assessed through a scale with a total score of nine points for three domains (selection of study group, comparability, and outcome). Studies were classified as having good, fair, or poor quality if the NOS score were six or larger, five or four, or less than four, respectively [27].
5. Statistical analyses
A fixed-effects meta-analysis was used to estimate pooled ORs and 95% CIs as the summary estimate. Heterogeneity was evaluated using the Q-statistics and I2 index score, with a significance set at I2 <50%. Potential publication biases were evaluated using the Egger’s test and the Begg’s funnel plot. Subgroup analyses were performed by various factors such as. Statistical analyses were carried out using STATA version 14.0 (Stata Corporation, College Station, TX, USA).
Results
1. Study selection
The search strategy found 430 references (PubMed: 233, EMBASE: 195, Hand search: 2). After duplicate studies (n=117) were removed, 313 studies were reviewed. Among the 313 studies, 198 studies were excluded base on title and/or abstract information. The remaining 115 studies were reviewed for eligibility based on the inclusion criteria. After a full text review, six studies were selected for the meta-analysis including a total of 806 subjects and 393 thyroid cancer cases <Fig. 1>.
2. Quality assessment
We assessed four studies using the Newcastle-Ottawa scale for quality assessment.
As shown in <Table 1>, two studies were classified as having good quality, while other four studies were classified a2020-07-15s having fair quality.
Table 1. Newcastle-Ottawa Scale Quality Assessment of the included studies (N=6)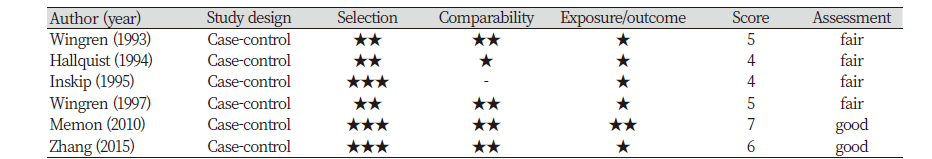
|
|
Study characteristics |
3. Study characteristics
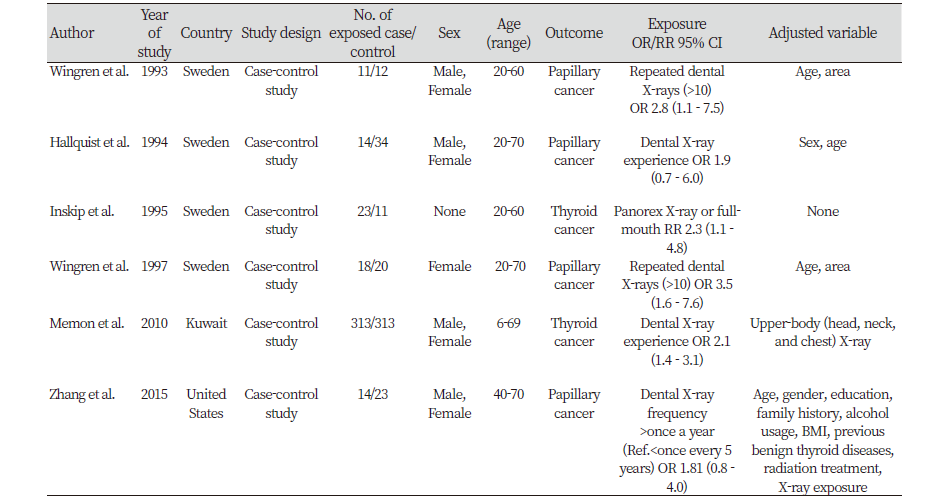
All included studies were case-control studies. These studies were published from 1993 to 2015. Studies were carried out in three countries: Four studies in Sweden, one in Kuwait, and one in the United States. One of the six studies was conducted on only women, but the other studies included both men and women. The studies specified either dental X-ray frequency or any experience in dental X-ray exam. The adjusted variable was most commonly the age variable, seen in four of the six studies.
4. Synthesis of results
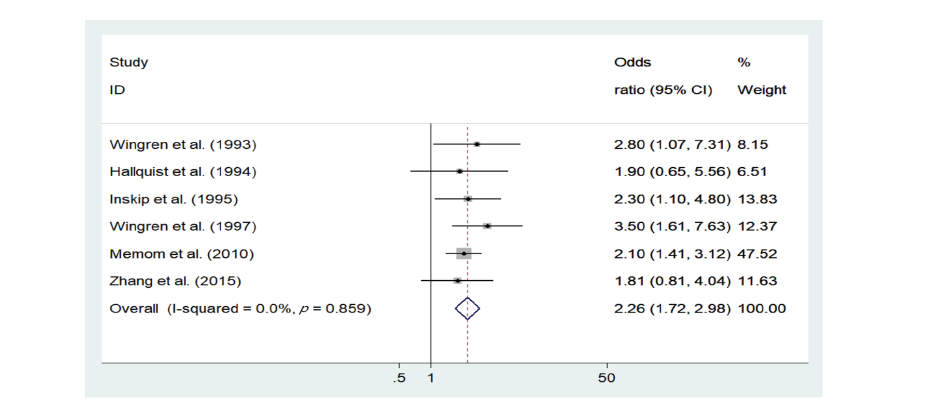
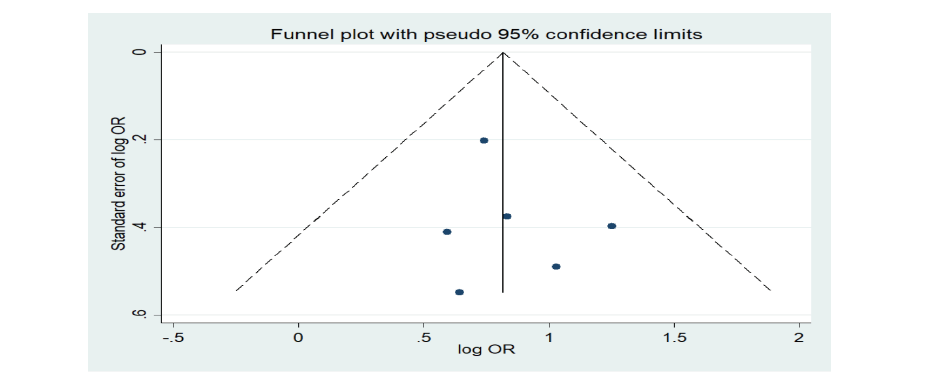
<Table 2> describes the primary outcome for each study. Results of the individual studies were presented in a forest plot<Fig. 2>. The fixed-effects model revealed that dental X-ray exposure was associated with increased thyroid cancer risk (OR = 2.26, 95% CI = 1.72-2.98). There was no heterogeneity in the data in the data (p=0.859, I2=0%). The Egger’s test (p=0.593) and Begg’s test (p=0.851) tests showed no publication bias <Fig. 3>.
5. Subgroup analyses
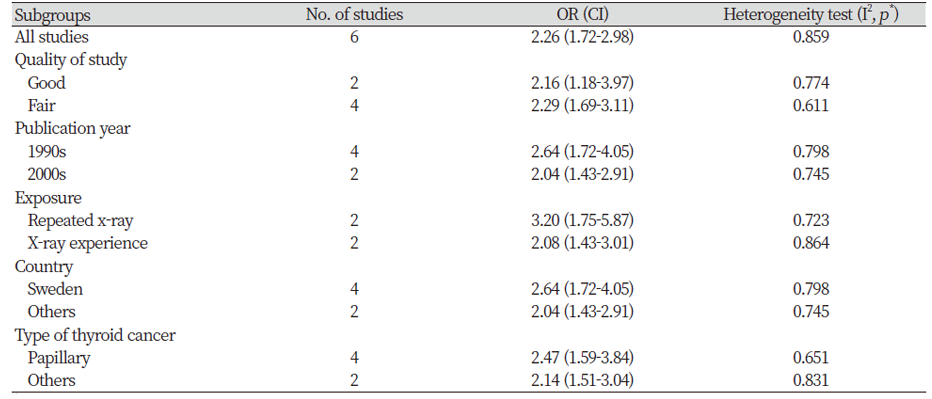
<Table 3> shows the subgroup analysis by quality of studies, publication year, X-ray exposure, countries where the study was conducted, and types of thyroid cancer. In two studies having good quality and four studies having fair quality, the summary OR were 2.16 (95% CI=1.18-3.97) and 2.29 (95% CI=1.69-3.11), respectively. By the publication year, the summary ORs were calculated as 2.64 (95% CI=1.72-4.05) among studies published in 1990s and 2.04 (95% CI=1.43-2.91) among studies published in 2000s. For repeated x-ray exposure and X-ray experience, the summary ORs were 3.20 (95% CI=1.75-5.87) and 2.08 (95% CI=1.43-3.01), respectively. When comparing different countries, the summary ORs were calculated as 2.64 (95% CI=1.72-4.05) in Sweden and 2.04 (95% CI=1.43-2.91) in other countries (Kuwait, United States). By types of cancer, the summary ORs were calculated as 2.47 (95% CI=1.59-3.84) among papillary cancer and 2.14 (95% CI=1.51-3.04) among other types of thyroid cancer. Sensitivity analysis was performed to evaluate the influence in which the meta-analysis was rerun repeatedly omitting each study at a time. All the results of the sensitivity analysis were similar to the current results (data not shown).
Discussion
The thyroid gland is sensitive to radiation carcinogenesis, and exposure to high ionizing radiation in childhood and adolescence is known to be the main environmental cause of thyroid cancer [28,29]. Although the risk due to diagnostic radiation, including exposure from dental X-rays, is considered minimal, the number of radiographic examinations can be gradually accumulated [30], and thus the risk of cancer can increase due to frequent exposure to radiation [31,32]. The current results showed that there was significant association between exposure to dental X-rays and thyroid cancer (OR=2.26, 95% CI=1.72-2.98), suggesting that exposure to dental X-rays may put patients at a greater risk for thyroid cancer than those without exposure. This study systemically screened relevant literature and included all case-control studies on dental radiation and thyroid cancer published as of September 2018. In addition, the heterogeneity and publication bias tests confirmed validity of the current meta-analytic results, including very low heterogeneity of the included studies and no publication bias.
While taking radiographs of the oral and maxillofacial areas, the thyroid gland is often exposed to radiation because it is either included in the irradiation area or located adjacent to the irradiation area. Particularly, the applied radiation dosage was the highest in the thyroid gland compared to other organs while taking radiographs of the apical area [33]. The comparison of the included studies in the current meta-analysis showed that the magnitude of risk tended to be higher in studies that assessed whether or not participants experienced “repeated” exposure to dental X-rays (OR=3.20, 95% CI=1.75-5.87), rather than any experience of dental X ray experience (OR=2.08, 95% CI=1.43-3.01). The result was consistent with previous studies, which reported that the risk of thyroid cancer increased with repeated dental radiation exposure [34]. Also, the current results were partially in accord with the results of previous studies that showed the association between exposure to dental radiation and increased risk of head and neck tumors [17,35]. Therefore, clinicians and health researchers should not overlook the risk of low radiation doses of dental X-rays and emphasize the importance of complying with safety regulations in order to protect the thyroid glands of patients during dental radiography.
However, the current results were not consistent with a previous meta-analysis, which found that the risk of meningioma due to dental radiation depended on the specific type of dental X-ray [27]. The association between thyroid cancer and different types of dental X-rays could not be examined in this study because most studies included in this meta-analysis reported only one type of dental X-rays. Future studies should compare the various types of dental X-rays in order to verify the possibility of specific X-ray being particularly more risky for thyroid cancer than others, rather than all kinds of X-rays being equally risky.
There are several limitations of this study. First, the current meta-analysis focused on case-control studies, and there may be a selection bias against other relevant studies with different research methods. Also, recall or memory bias may have influenced this study due to the use of self-report assessments in the included studies. Next, the number of studies included in this meta-analysis was small due to a lack of relevant research on this topic, the quality of the included studies was not good. Finally, there was a lack of information about the radiation exposure dosage, dental X-ray type, and age of the study participants in the included studies, which limits our capability of conducting related subgroup analyses. Therefore, future studies should report detailed information (e.g., radiation type, exposure dose) on both the dental radiation and detailed demographic information of study samples (e.g., education and health status) in order to contribute to better understanding of the mechanisms underlying the association between dental radiation and thyroid cancer. Despite those limitations, this study has important implications in that it provided further evidence that low-dose ionizing radiation by dental X-rays may be associated with increased risk of thyroid cancer.
Conclusions
We conducted a meta-analysis of the case-control studies, which evaluated the association between exposure to dental radiation and the risk of thyroid cancer.
1. The current results suggest that there may be an association between dental X-rays and increased thyroid cancer risk.
2. Future studies are needed to compare the impacts of different degrees and types of dental X-ray exposure on thyroid cancer risk and to identify ways to improve protection guidelines for dental radiation.