Introduction
Dental caries is a chronic disorder caused by plaque accumulation that leads to irreversible tooth damage and loss [1]. Despite its decreasing incidence, dental caries remains an oral disease with the highest prevalence rate across all age groups [2,3]. Bacterial, host, and diet factors contribute to dental caries [4]. Of these, bacteria are the most prominent cause of dental caries [5]. Bacteria attach to tooth surfaces, where they proliferate and aggregate to form dental plaque. Organic acids from carbohydrates retained in dental plaque induce demineralization and consequently dental caries in the long term [5,6].
Over 700 bacterial strains contribute to dental plaque formation [7]. Streptococcus mutans is known to be the most cariogenic causal factor of dental plaque [8,9]. S. mutans produces highly concentrated organic acids thereby directly inducing tooth demineralization and playing a crucial role in dental plaque formation because S. mutans forms viscous polysaccharides such as glucan [10,11]. Therefore, preventing and eliminating dental plaque by inhibiting S. mutans proliferation is crucial for preventing dental caries [12].
Toothbrushing and flossing are the most basic methods for manual removal of dental plaque and preventing dental caries [13,14]. If not performed correctly, toothbrushing may fail to effectively remove dental plaque [15]. Furthermore, the anatomical characteristics of tooth pits and fissures make it difficult for bristles of a toothbrush to reach the occlusal and buccolingual surfaces [16]. To overcome this limitation, toothpaste or mouthwash products containing antimicrobial agents that inhibit bacterial growth and colonization have been used. However, these products contain large amounts of synthetic chemicals that can induce side effects such as tooth discoloration, a burning sensation, and dry mouth [17-19]. There is a growing need for safe antimicrobial agents that effectively remove oral bacteria.
Major antimicrobial substances include natural substances such as xylitol, mastic, and essential oils [20]. Active research is being conducted on electrolyzed water produced by electrolyzing water after its stability and antimicrobial effect were recently verified [21-23]. Electrolyzed water is produced by passing an electric current through water and contains over 300 ppb dissolved hydrogen [24]. Physical and chemical properties of electrolyzed water depend on the type of metal electrode used and ions added and on whether a membrane was used during electrolysis [25]. Although the efficacy of electrolyzed water depends on its physical and chemical properties, it is reported that electrolyzed water has anticancer, antioxidative, and antimicrobial effects [23,26,27]. Electrolyzed water has fewer side effects as compared to synthetic chemicals and is simpler and cheaper to use as compared to natural substances. This study was aimed at demonstrating the inhibitory effect of electrolyzed water on S. mutans proliferation and dental plaque formation and at obtaining basic data that can be used to develop oral supplements for preventing dental caries.
Methods
1. Methods
1) Bacterial culture
Freeze-dried S. mutans KCOM 1045 obtained from the Korean Collection for Oral Microbiology was inoculated on a Mitis Salivarius-Bacitracin (MSB) agar plate (Mitis Salivarius Agar, Difco Laboratories, MD, USA) containing 15% of sucrose (Duksan, Korea) and 0.2 U/mL bacitracin (Sigma Chemical, MO, USA) and cultured at 37℃ in a 5% CO2 incubator (Sanyo, Japan) for 48 hours. One of the several S. mutans colonies that formed on the MSB agar plate was inoculated into brain heart infusion (BHI, Difco Laboratories, MI, USA) broth and cultured for 24 hours under the same conditions. The cultured bacteria were then subcultured and used in the experiment.
2) Electrolyzed-water preparation
500 mL of 20℃ tap water was processed in Natural Gargle Plus (EBIOMW-100, Ebioteco, Korea) for 1 minute to produce neutral electrolyzed water (1.2–1.3 ppm dissolved hydrogen, oxidation-reduction potential of 500 mV, and pH 7.2). The electrolyzed water was filtered using a 0.2 μm syringe filter (Minisart, Sartorius Stedim, Germany) and used in the experiment.
3) Treatment with electrolyzed water
S. mutans cultured in BHI broth for 6 hours was centrifuged at 4℃ and 7,500 rpm for 15 minutes to precipitate the bacterial cells. After removal of the supernatant, the bacterial pellet was washed twice with 5 mL of phosphate-buffered saline (PBS), and 5 mL of electrolyzed water was added to the pellet. 45 mL of PBS was added immediately after exposing the sediment to electrolyzed water for 1 (group EW1) or 3 minutes (EW3). The solution was then centrifuged at 7,500 rpm for 15 minutes and evenly resuspended on 50 mL of BHI broth. Tap water (TW) was used as the negative control, and Listerine (Listerine, Korea Johnson & Johnson, Korea) was used as the positive control.
4) Analysis of S. mutans proliferation
The S. mutans suspension exposed to electrolyzed water was serially diluted 10-fold down to 1:10−7; 150 μL of the suspension was inoculated on MSB agar. Colonies were counted after 48 hours of incubation. The experiment was repeated three times.
5) Analysis of dental plaque formation by S. mutans
Dental plaque formation induced by S. mutans was analyzed using O’Toole’s dental plaque formation assay [28]. First, each well of a six-well polystyrene plate was coated with artificial saliva (Xerova solution, Kolmar Korea, Korea) and inoculated with 2 mL of the S. mutans suspension exposed to electrolyzed water. The plate was then incubated for 24 hours. The supernatant was removed from each well, and the wells were washed 3 times with 2 mL of PBS to remove any remnant bacteria from the plate. Each well was treated with 1 mL of 0.2% crystal violet solution for 30 minutes to stain the dental plaque. The plate was washed five times with PBS and treated with 10% acetic acid to extract the dye; 200 μL of the extracted dye was added into each well of a 96-well plate, and optical density was measured at 570 nm (OD570) (ELISA, ASYS, Austria). The experiment was repeated three times.
2. Statistical analysis
Obtained data were analyzed using IBM SPSS (IBM 25.0 for Windows, SPSS Inc, Chicago, IL, USA) with the level of statistical significance at 5%. Since the data did not show a normal distribution in the Shapiro–Wilk test, all data were analyzed using nonparametric tests. The results regarding S. mutans proliferation and dental plaque formation following the treatment with electrolyzed water were analyzed using the Kruskal–Wallis test in addition to Wilcoxon’s rank sum test for post hoc analysis. The level of statistical significance was corrected using the Bonferroni test during the post hoc analysis.
Results
1. Effect of electrolyzed water on S. mutans proliferation
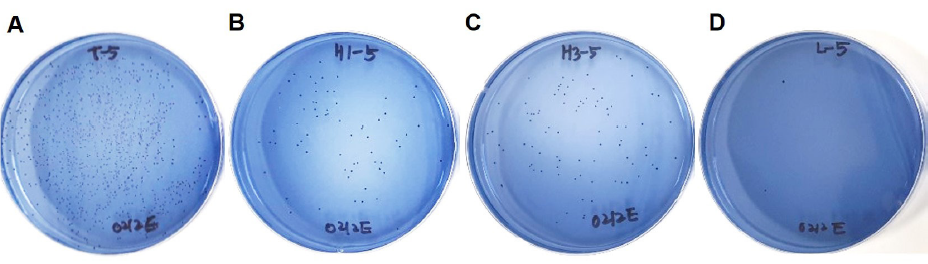
MSB agar plates were grossly examined to compare the extent of S. mutans colony formation. Colony formation was clearly reduced for S. mutans exposed to electrolyzed water as compared to that exposed to tap water. No clear difference in the extent of colony formation was observed between the exposure time points (1 or 3 minutes) <Fig. 1>.
The actual count of S. mutans colonies exposed to tap water was 2.43×109 ±1.57×109 and that of S. mutans exposed to electrolyzed water was 3.00×107±1.16×107 after 1 minute of exposure and 4.70×107±1.08×107 after 3 minutes of exposure. The colony count was significantly lower for S. mutans exposed to electrolyzed water as compared to that exposed to tap water (p<0.001). However, no significant difference in the colony count was found between exposure time points (p >0.05). In the Listerine group (positive control), the colony count was 4.00×106 ±2.41×106, which was significantly lower than the colony counts in all other groups (p<0.05) <Table 1>.
2. Inhibitory effect of electrolyzed water on dental plaque formation by S. mutans
The amount of dental plaque formed by S. mutans was analyzed using the dental plaque formation assay. The amount of dental plaque was 0.79±0.02 for group after tap water exposure, 0.38±0.01 after 1 minute of exposure, and 0.13±0.00 for after 3 minutes of exposure. S. mutans exposed to electrolyzed water formed significantly less dental plaque as compared to S. mutans exposed to tap water (p<0.05). Additionally, dental plaque formation significantly decreased as exposure time increased from 1 to 3 minutes (p<0.05). S. mutans treated with Listerine (positive control) showed significantly reduced dental plaque formation compared to all other groups (p<0.05) <Table 2>.
Discussion
While the antimicrobial effect of electrolyzed water against various microorganisms is being verified through research and electrolyzed water is being used in various fields for its antimicrobial effect, research on its effect on oral bacteria is lacking. This study was aimed at assessing the effect of electrolyzed water-produced by membraneless electrolysis of tap water-on S. mutans to determine whether it could be used to prevent dental caries.
In the analysis of the effect of electrolyzed water on S. mutans proliferation, electrolyzed water significantly inhibited S. mutans proliferation as compared to tap water. This result is consistent with previous studies reporting that electrolyzed water has antimicrobial effects against various bacterial strains [22,23]. Lee and Choi [23] cultured five strains of periodontal bacteria on plate media after exposing them to electrolyzed water and observed a clear reduction in bacterial proliferation as was also observed in this study. Additionally, they reported a significant reduction in the number of bacteria in the saliva of subjects who rinsed their mouth with electrolyzed water. Lee [22] observed significant inhibition of S. mutans and S. sobrinus growth following 30 seconds or 1 minute of exposure to electrolyzed water. The electrolyzed water used by Lee contained NaCl. Numerous studies that reported electrolyzed water to have potent antimicrobial effects used electrolyzed water containing a small amount of NaCl. Electrolyzed water containing NaCl has an excellent antimicrobial effect because it is highly acidic, has high oxidation-reduction potential, and contains high concentrations of hypochlorous acid and free chlorine [29]. Although the electrolyzed water used in this study was neutral hydrogen water produced by electrolyzing pure tap water, it clearly inhibited S. mutans proliferation. Although the electrolyzed water was not as effective as Listerine (used as the positive control) in inhibiting bacterial growth, its antimicrobial effect can be improved by increasing electrolysis duration and intensity according to Nakajima et al. [30]. Nakajima et al. [30] reported that the antimicrobial effect of tap water contaminated with four strains of gram-positive and negative bacteria increased as electrolysis time and intensity increased. Since free chlorine is responsible for the antimicrobial effect of electrolyzed water without NaCl, the antimicrobial effect of electrolyzed water can be enhanced by increasing electrolysis time to increase the concentration of free chlorine [30]. Electrolyzed water produced with appropriate electrolysis duration and intensity may effectively inhibit S. mutans proliferation without the addition of NaCl.
While the reduction in the S. mutans count is meaningful progress towards dental caries prevention, even a small population of remaining S. mutans can significantly contribute to the development and progression of dental caries since S. mutans is highly pathogenic. Dental plaque that forms on top of the viscous polysaccharides produced by S. mutans protects bacteria and free acids, thereby accelerating tooth demineralization; for this reason, inhibiting dental plaque formation is highly important for preventing dental caries [10-12].
In this study, electrolyzed water effectively inhibited S. mutans proliferation and dental plaque formation. This result is consistent with a study by Kim et al. [21] who reported exposure time-dependent inhibition of dental plaque formation by S. mutans and S. sobrinus as well as clear downregulation of the gtf and gbpC genes involved in dental plaque formation. Since genetic analysis was not performed in the present study unlike the study by Kim et al. [21], the possibility that the observed inhibition of dental plaque formation was simply due to the reduction in the S. mutans count cannot be ruled out. Additionally, this study has the inherent limitations of laboratory research. Although the mechanism of the antimicrobial and antiplaque effects of electrolyzed water could not be thoroughly elucidated due to these limitations, the results of previous studies [21-23,29,30] and the present study suggest that the free chlorine produced during the electrolysis of tap water destroys bacterial cell membranes and induces the aggregation of intracytoplasmic proteins thus inhibiting bacterial growth. Additionally, the hydrogen molecules that pass through the cellular membrane inhibit the expression of genes associated with dental plaque formation thereby inhibiting dental plaque formation.
Although this study does not elucidate the mechanism by which electrolyzed water prevents dental caries, it demonstrated that electrolyzed water produced from pure tap water can inhibit S. mutans proliferation and dental plaque formation, confirming the suitability of electrolyzed water as an oral care product. Unlike existing oral care products, electrolyzed water has no side effects, is easy to prepare, and is practical. With further research elucidating the anticaries mechanism of electrolyzed water using various laboratory techniques including genetic analysis and assessing the anticaries effect of electrolyzed water in clinical settings, electrolyzed water may be employed to develop an excellent anticaries agent to be used by those struggling with oral care.
Conclusions
In this study, S. mutans, a major causal bacterial pathogen of dental caries, was treated with electrolyzed water produced via membraneless electrolysis of tap water, and the following results were obtained.
1. Electrolyzed water significantly inhibited S. mutans proliferation (p<0.001).
2. Electrolyzed water inhibited dental plaque formation by S. mutans (p<0.001). Dental plaque formation was significantly more inhibited as the duration of exposure to electrolyzed water increased (p<0.05).
These results demonstrate that electrolyzed tap water can effectively prevent dental caries by inhibiting S. mutans proliferation and dental plaque formation by S. mutans.