Introduction
The oral health of older people is one of the very basic factors required for maintaining a healthy life. Oral functions, including chewing, swallowing, and pronunciation, are essential for maintaining social interactions as well as sound quality of life [1]. In particular, oral infection prevention and oral function improvements are necessary in order to masticate food, enjoy flavours, and display diverse expressions [2]. However, the mouths of elderly individuals often show substantial tooth loss due to oral infections that have accumulated throughout their lives [3]. Furthermore, remaining teeth are often worn out on the occlusal surface due to dental attrition, which results in lower cusps and decreased mastication [4]. In addition, decreased tongue strength, mobility and decreased saliva secretion due to salivary gland aging make swallowing difficult to the elderly [5]. With age-related decreased swallowing, the risk of aspiration (food or saliva entering the airway instead of the oesophagus) increases [6]. If these difficultes persist for a long time, the risk of aspiration pneumonia also increases [7]. Therefore, active efforts are required to improve the factors that negatively affect the quality of life for older people related to their oral health. These include conditions such as decreased chewing, dry mouth, and decreased swallowing [6,7].
The methods for improving the oral health of older people include oral health education, preventative assessments, and early treatment [8]. However, oral health education, which provides the basis for oral health motivation, is less effective due to fewer individuals carrying out the required behavioural changes. Care by experts, preventative assessments, and early treatment also is less effective because they require visits to the oral health centre [8,9]. Therefore, an oral exercise program that can be easily followed in daily life will effectively improve the oral health of older people [10].
In Japan, which has a high life expectancy, oral health improvement methods were added to oral health-related programs through oral health education [11]. Japan has an oral exercise policy for improving oral health [9] that has been reported to be very effective in improving the oral health of older people in an aging and super-aging society [9,11,12]. Furthermore, the recommended exercises can be continuously used by older people without complications, financial burden, or limitations of time or place [13].
An oral exercise program comprised of saliva-producing, chewing-strengthening, swallowing-strengthening, and speaking-strengthening exercises was also developed and applied in older people in facilities throughout Korea. As a result, this program has improved the level of oral health in elderly individuals with healthy oral functions [14]. The program promoted the active recovery, maintenance, and improvement of oral functions in elderly individuals with decreased oral functions. Altogether, it increased the quality of life and oral health of older people [15].
Furthermore, it has been reported that whole-body exercises have aging prevention and health improvement effects in older people [16,17]. These positive effects of whole-body exercises in older people include physiological and affective elements related to decreased depression and anxiety [18] as well as social interactions such as the maintenance of bonding with others. In this respect, whole-body exercises can be considered an essential activity in old age [19]. About 40% of people aged 60 years or older experience discomfort from dysphagia, and this is related to general muscle weakening [20]. Furthermore, whole-body muscle weakening affects muscles of the head and neck as well, and weakening of the tongue muscles is the root cause of dysphagia [21,22]. Therefore, whole-body exercise may have a positive effect on oral health improvements in older people. It is anticipated that oral function improvements will be greater in elderly individuals who perform oral exercises with whole-body exercises than in older adults who only perform oral exercises.
Therefore, an oral exercise program was selected as the oral function improvement methodology of this study. To differentiate our investigation from previous studies, we studied oral function improvements that result from oral exercise combined with whole-body exercise.
Methods
1. Research design
This study applied the oral exercise program with whole body gymnastics to improve oral function to elderly subjects over 65 years of age, and used a non-equivalent pre-post similar experimental design to confirm the effect.
2. Participants
This study was designed as a case-control, three-arm randomized clinical trial. Participants were elderly individuals (aged ≥65 years) from three centres for senior citizens in Gyeonggi-do, Korea. Individuals with a history of temporomandibular joint dysfunction, oral and maxillofacial surgery, diagnosis of mastication and neck movement limitations, myocardial infarction, and stroke were excluded.
Participant recruitment began on May 1, 2019 and lasted until Dec 31, 2019. The participants were randomly assigned to intervention group I (only oral exercise), intervention group Ⅱ (oral exercise with whole-body exercise), and the control group (no intervention), by drawing consecutively numbered envelopes.
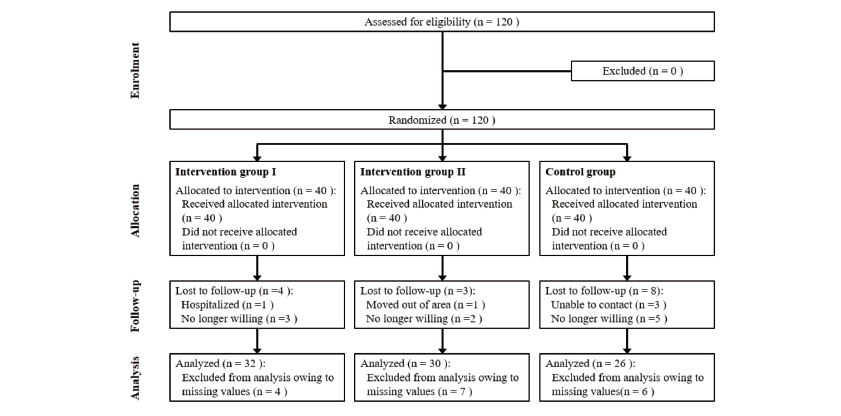
The required sample size was calculated as 92 individuals using G*power 3.1 program with effect size (d) of 0.25, significance level (α) of 0.05, and test of power (1-β) 85%. The effect size was estimated in reference to a previous study that used an effect size of 0.25 [23]. Considering drop-out, 40 participants were selected for each group, with a total of 120 participants. After excluding those who terminated their participation during the study, data from a total of 88 participants (32 participants in the intervention group I, 30 in intervention group Ⅱ, and 26 in the control group) were analyzed <Fig. 1>.
This study was conducted after obtaining approval from 00 University’s Institutional Review Board (SHIRB-201902-HR-087-02-(2)). Written consent forms were obtained from the research participants prior to their participation in the study. The study was conducted in full accordance with ethical principles, including the World Medical Association Declaration of Helsinki (version 2008), and the clinical trial was registered with the Clinical Research Information Service of the Korean government (KCT0004827–08/03/2020).
3. Intervention
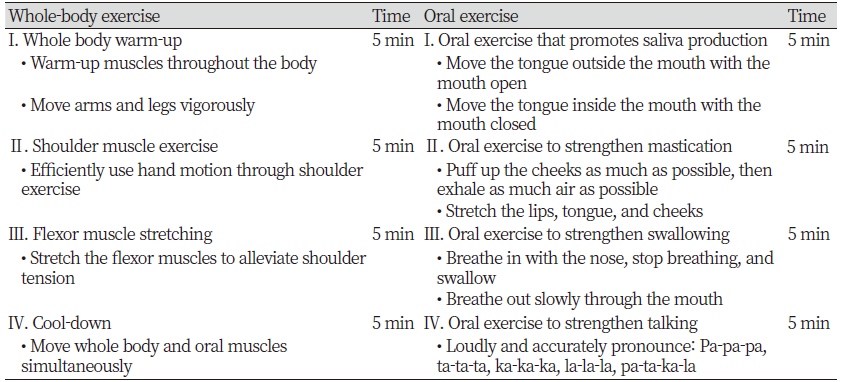
Detailed information regarding the whole-body exercise and oral exercise program is listed in <Table 1>. The whole-body exercises were performed by controlling breathing and muscle strength in synchrony with a song with a 4/4 rhythm. Oral exercises were applied by referencing the elderly oral function improvement exercises that were developed by a health clinic in Kochi city, Japan [15] and the Korean program [14] For the intervention group I, the oral exercise program was conducted twice a week for five weeks. The participants in the intervention group Ⅱ performed the oral exercise program with the whole-body exercises twice a week for five weeks.
4. Outcome measures
The general characteristics of the study subjects were sex, age, educational level, economic status, and spouse living together. As for the characteristics of oral health-related behavior, the Frequency of tooth-brushing, tongue cleaning, gum cleaning, use of oral hygiene products, and Scaling during the previous year.
The Oral Health Impact Profile-14 (OHIP-14) [24] was collected through self-reported surveys. OHIP-14 is comprised of a total of 14 questions related to the condition of oral health in the past year. Each item is answered on a 5-point Likert scale. The maximum total score is 70 points, with higher scores indicating better oral health related quality of life. In addition, the Geriatric Oral Health Assessment Index (GOHAI); [25] which is comprised of 12 items, was used to investigate the oral health status of older people over the past three months, with higher scores indicating better oral health related quality of life.
To measure the unstimulated saliva flow rate, the participants were advised not to take actions that could promote oral functions (talking, eating, drinking, smoking, or oral hygiene) for two hours before collecting saliva. The participants swallowed all the saliva that was in their mouths immediately before the saliva was collected. Then, their saliva was collected in a cup for five minutes. Because this study aimed to identify the changes in the amount of saliva with respect to each exercise program rather than diagnose xerostomia, the amount was measured in weight (g) rather than volume (mL). For this, an ultra-precision electronic scale (Model: BJ-420, Manufacturer: SHINKO) was used. As unstimulated saliva flow rates can differ depending on an individual’s overall health status each day, measurements were taken twice, at identical times and places, and the mean value was calculated.
The Iowa Oral Performance Instrument (IOPI)(IOPI Medical, WA, USA) is a portable device for measuring the pressure of anatomical structures in the mouth [26]. This device was used to measure tongue and cheek strength three times, consecutively, and the maximum value (KPa) was recorded. While taking the measurements, a one-minute rest was provided between each exercise to alleviate orofacial muscle fatigue, and all measurements were made by one researcher.
To measure anterior tongue strength, the examiner fixed the bulb so that the alveolar ridge of the hard palate and the region that was 10 mm from the beginning of the tongue were in contact. To measure posterior tongue strength, the examiner fixed the bulb so that the back part of the hard palate and the tongue touched. The participants were subsequently asked to press the bulb toward the hard palate with their tongue, as forcibly as possible, for two seconds. To measure cheek strength, the bulb was placed between the teeth and cheeks, and the participants were asked to softly close their mouth. After placing the bulb in the anterior region of their cheeks, the participants were asked to apply pressure to the bulb, with as much strength as possible, for two seconds.
5. Data analyses
The data collected in this study were analyzed using SPSS Statistics (version 22.0, IBM Corporation, New York, NY, USA), with a statistical significance level of 0.05. To compare the general characteristics and oral health-related behaviours of the research participants, a chi-square test was performed. The ANOVA test was performed to compare the average values after the program of the three groups. To identify the changes that occurred after the program’s completion among the three groups, a paired t-test was performed.
Results
1. General characteristics
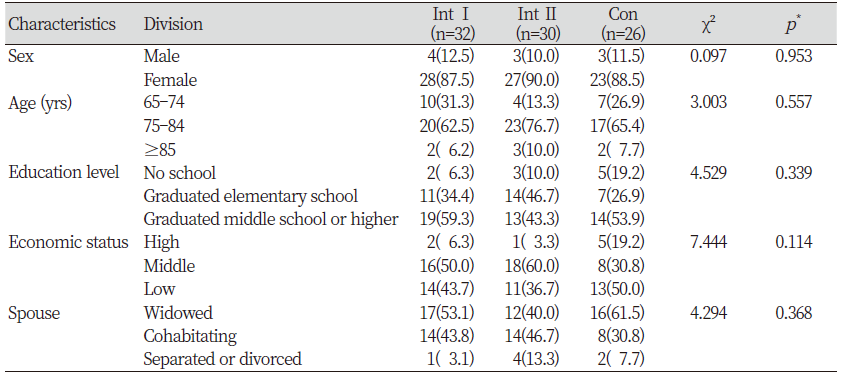
Sex, age, education level, economic status, and spousal cohabitation were recorded as general characteristics of the research participants. There were no statistically significant differences in these factors among the three groups, showing homogeneity between intervention group I, intervention group Ⅱ, and the control group<Table 2>. In all three groups, the proportion of female participants was higher than that of male participants, and the 75–84-year age groups contained the greatest participant populations.
Table 2. Divisions and contents of the questions
|
|
*by chi-square test Int I; Intervention group I, Int Ⅱ; Intervention group Ⅱ, Con; Control group |
2. Oral health-related behaviors
The frequency of tooth-brushing, tongue cleaning, gum cleaning, use of oral hygiene products, and scaling in the past year were investigated as factors related to oral health-related behaviours of the research participants. There were no statistically significant differences among the three groups, thereby demonstrating homogeneity among the three groups <Table 3>.
Table 3. Oral health-related behaviors Unit : N(%)

|
|
*by chi-square test Int I; Intervention group I, Int Ⅱ; Intervention group Ⅱ, Con; Control group |
3. Effects of the oral exercise program with whole-body exercise
The findings of the oral function improvement investigations across the two measurements, before and after the program, are as follows <Table 4>. OHIP-14 and GOHAI increased in both intervention groups; however, these increases were not statistically significant. The between-group comparisons of the post-intervention measures also did not show a difference.
Saliva flow rate increased by 0.81 in intervention group Ⅰ and by 0.42 in intervention group Ⅱ, and these changes were statistically significant. Furthermore, the between-group comparisons also showed that intervention group Ⅰ had a greater increase than the other groups (p<0.01).
Anterior tongue strength measured with IOPI increased by 3.28 in intervention group Ⅰ and by 8.36 in intervention group Ⅱ, and these changes were statistically significant. Additionally, the between-group comparisons also showed that intervention group Ⅱ had a greater increase than the other groups (p<0.05). Posterior tongue strength measured with the IOPI increased by 1.93 in intervention group I, however this increase was not statistically significant. Posterior tongue strength also increased by 8.03 in intervention group Ⅱ, with statistical significance. Furthermore, between-group comparisons also showed that intervention group Ⅱ had a greater increase in posterior tongue strength than did the other groups (p<0.05). Cheek strength, as measured with the IOPI, increased by 3.09 in intervention group I, without statistical significance, and by 9.00 in intervention group Ⅱ, with statistical significance. Furthermore, between-group comparisons showed that the increase in cheek strength in intervention group Ⅱ was greater than that in the other groups (p<0.01). The control group showed changes that were not statistically significant in the various measurements.
Discussion
This study developed a program that combined whole-body exercises with existing oral exercises and identified related oral function improvements in elderly individuals. Scores of the OHIP-14 and GOHAI evaluations, which are the two representative indicators for measuring the relationship between oral health and quality of life, increased for the intervention groups, without statistical significance. This may be because the program was only five weeks in duration, which could have been too brief a period for individuals to consider that their quality of life improved as a result of their oral health. However, the participants’ behavioural changes could become significant when the program is continuously followed. Thus, further long-term measurements and evaluations are required in the future.
Saliva is responsible for keeping the mouth healthy by providing moisture to the oral mucous membranes and to prevent drying [5]. In the mouths of older people, the oral mucous membranes contract and saliva secretion decreases, resulting in xerostomia. Pain in the oral mucous membrane can decrease oral functions, such as mastication, deglutition, and pronunciation, and increase tongue coating, which leads to halitosis [6,7]. The findings of this study showed that the saliva flow rate did not change significantly in the control group, while the individuals in the intervention groups presented significant increases. In particular, the saliva flow rate of intervention group Ⅰ increased from 1.12 to 1.94, and this change was more pronounced than those of the other groups. Between-group comparisons also showed statistically significant differences. It has been reported that oral exercises are effective at improving xerostomia and increasing unstimulated saliva flow rates [14]. If appropriate, oral function exercises that are performed before further health deterioration and the functional decline in the salivary glands that accompanies aging and drug complications, can be modified [5]. In conclusion, increased saliva flow rates were found to result only from oral exercises, and it is thought that continuous oral exercise training among older people will have a positive impact on oral functional improvements.
The tongue is a stomatognathic structure that is comprised of various intrinsic and extrinsic muscles [27]. Extrinsic muscles play a role in changing the location of the tongue through protrusion, retraction, depression, and elevation, while intrinsic muscles play a role in changing the shape of the tongue [28]. These muscles cooperate during the deglutition process by regulating the mastication, formation, and manipulation of food in the mouth, and they are also involved in breathing and language [29]. Furthermore, cheek muscles reside in the lower interior location, and the buccinator muscle is the agonist muscle that plays a role in ensuring that food is not left between the teeth and gums (i.e. sulcus lateralis) through continuous contraction during mastication [26]. In this study, the tongue and cheek strength measured with the IOPI showed that anterior tongue strength increased significantly and posterior tongue strength and cheek strength increased without statistically significant differences in intervention group I. However, in intervention group Ⅱ, anterior tongue strength, posterior tongue strength, and cheek strength significantly increased, and the between-group comparisons showed statistically significant differences. In relation to this, previous studies have also implied that oral exercise can improve oral strength, including mastication, the buccinator muscle, and the root of the tongue, due to movement of the oral muscles [26]. Prior studies have also showed that long-term participation in oral exercises with physical activities can inhibit the deterioration of oral functions in older people and contribute to the maintenance of oral health [11]. Tongue and cheek strength were found to significantly improve in this study as well as being partially in line with the findings of previous studies [26]. Furthermore, although it was difficult to clearly hypothesize the reason for the more significant increase in oral strength when whole-body exercises were performed with oral exercises, it may be that relaxation of the body and tense muscles through whole-body exercises can increase blood flow to the brain [30] and increase the effects of oral exercise. Therefore, it is necessary to identify the cause for the observed improvements through future studies.
The findings of this study showed that the saliva flow rate increases with oral exercise alone and that improvements in the saliva flow rate and oral strength were observed when whole-body exercises and oral exercises were performed in parallel. However, because the duration of the study was five weeks, there was not enough time to test the effects of oral exercise with whole-body exercises, and this likely contributed to the lack of large differences between the two groups. Previous research has implied that whole-body exercise with oral exercise contributes to oral function improvements [11]. Considering these findings, it is anticipated that the long-term application of an oral exercise program with whole-body exercise will lead to large oral function improvements.
Although there have been many studies focused on oral exercises as methods for preventing xerostomia in older people, no investigations have used an oral exercise program in combination with whole-body exercise to improve oral functions. Furthermore, no studies have measured the oral strength using IOPI. Thus, there are difficulties with directly comparing the findings of this study to those of prior studies. This study is significant in that it pioneered an investigation of oral functional improvements for older adults with insufficient prior research. This study also compared the differences in the oral functional improvements of regular oral exercise with oral exercise in combination with whole-body exercise. It is thought that an oral exercise program with whole-body exercise will increase the oral health related quality of life of older people and contribute to oral health improvements. This combined program will benefit an aging society.
Conclusions
To evaluate the impact of an oral exercise program with whole-body exercise on the oral function of older people. The participants (aged ≥65 years) were divided into three groups: intervention group Ⅰ (only oral exercise), intervention group Ⅱ (oral exercise with whole-body exercise), and control group. Before and after the program, the oral health status, saliva flow rate, and oral muscle strength were measured. Analyses were performed to compare the three groups and to identify changes in the three groups before and after the program.
1. Saliva flow rate increased in intervention group Ⅰ and in intervention group Ⅱ. These increases were statistically significant.
2. In oral strength measurements using the IOPI, anterior tongue strength increased significantly in intervention group I. In this group, posterior tongue and cheek strength increased; however, these differences were not statistically significant.
3. In intervention group Ⅱ, anterior tongue, posterior tongue, and cheek strength increased, and these changes were statistically significant.
The oral exercise program with whole-body exercise was found to have positive effects on increasing the saliva flow rate and oral strength. No significant differences were observed in the quality of life related to oral health. Therefore, in subsequent studies, repeated studies are needed to confirm changes in oral health quality of life through long-term measurement.
Acknowledgements
This study was funded by the National Research Foundation of Korea and supported by the 2019 Ministry of Science and Information Communication Technology (No. 2018R1A2B6006701). The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.