1Apple Tree Institute of Biomedical Science, Apple Tree Medical Foundation
2DOCSmedi Co., Ltd.
Correspondence to Hye-Sung Kim, Apple Tree Institute of Biomedical Science & Apple Tree Dental Hospital, Apple Tree Medical Foundation, 1450 Jungang-ro, Ilsanseo-gu, Goyang-si, Gyeonggi-do, 10387, Korea. Tel: +82-031-913-9000, E-mail: hyesungk2008@appleden.com
Correspondence to In-seong Hwang, Apple Tree Institute of Biomedical Science & Apple Tree Dental Hospital, Apple Tree Medical Foundation, 1450 Jungang-ro, Ilsanseo-gu, Goyang-si, Gyeonggi-do, 10387, Korea. Tel: +82-031-913-9000, E-mail: ghkddlstjd@appleden.com
Volume 25, Number 2, Pages 101-11, April 2025.
J Korean Soc Dent Hyg 2025;25(2):101-11. https://doi.org/10.13065/jksdh.2025.25.2.2
Received on February 05, 2025, Revised on March 18, 2025, Accepted on April 03, 2025, Published on April 30, 2025.
Copyright © 2025 Journal of Korean Society of Dental Hygiene.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(http://creativecommons.org/licenses/by-nc/4.0)
Objectives: Probiotic supplementation, integrated into routine oral care, may improve periodontal status and metabolic function. In this retrospective longitudinal study, we evaluated the efficacy of probiotics added to non-surgical periodontal treatment (NSPT) in reducing the burden of oral pathogens and improving metabolic dysfunction. Methods: Twenty outpatients with chronic periodontitis and at least one metabolic abnormality underwent NSPT with daily probiotic supplementation for 12 weeks. Baseline and post-intervention assessments included clinical indices, blood pressure, and blood biomarkers (HbA1c, fasting glucose, and lipid profiles), with analysis of oral pathogens using qPCR. Results: Among the 13 eligible participants, probiotics with NSPT resulted in significant reductions in HbA1c (-0.3%, p<0.001), and systolic blood pressure (-9.6 mmHg, p<0.001), and Tannerella forsythia (p<0.05) levels, along with decreasing trends in other pathogens. However, no significant changes were noted in periodontal indices, including probing pocket depth and bleeding on probing, nor in lipid profiles. Conclusions: These f indings suggest that probiotic supplementation with NSPT may improve glycemic control and systolic blood pressure but may have a limited effect on periodontal indices, warranting larger trials to confirm its potential.
Blood glucose, Blood pressure, Periodontal therapy, Periodontitis, Probiotics
Metabolic syndrome is characterized by the presence of any three out of five criteria: fasting blood glucose (FBG), increased waist circumference, elevated triglycerides (TG), reduced high-density lipoprotein cholesterol (HDL-C), and elevated blood pressure [1]. Inadequate management of these risk factors can lead to the development of chronic systemic diseases [1]. Optimal management of these risk factors primarily involves dietary changes to improve metabolic health. Emerging evidence highlights the central role of diet in modulating microbiota composition [2], while the oral-gut microbial axis has been shown to influence systemic immunomodulation [3,4]. In this context, probiotics appear to modulate the immune system through multiple mechanisms including competition for nutrients and adhesion sites in the gut, interference with colonization of pathogens, production of antimicrobial compounds like bacteriocins, alteration of colonic pH, and non-specific stimulation of host immune responses [5]. Furthermore, studies have demonstrated that probiotic consumption enhances gut microbiota composition and homeostasis, thereby reducing the production of reactive oxygen species (ROS) and inflammatory metabolites, which subsequently decreases the risk of systemic inflammation.
A recent systematic review and meta-analysis showed that probiotic supplementation can reduce the levels of cardiovascular risk factors, such as body mass index (BMI), FBG, and low-density lipoprotein cholesterol (LDL-C) in patients with metabolic syndrome [6]. In mild periodontitis, non-surgical periodontal treatment (NSPT) may worsen coronary artery disease and metabolic syndrome control [7]. Given this association, restoring the microbial balance in the oral cavity and the gut through NSPT combined with adjunctive probiotic therapy may positively impact systemic inflammation and, consequently, improve metabolic health outcomes.
Chronic periodontitis is a multifactorial inflammatory disease characterized by progressive destruction of the tooth-supporting structures, and affects a significant portion of the adult population worldwide [8]. In particular, periodontitis is increasingly recognized as a comorbid condition associated with several chronic systemic diseases, including diabetes mellitus (DM), cardiovascular disease, metabolic diseases, rheumatoid arthritis, certain cancers, respiratory diseases, and cognitive disorders including Alzheimer’s disease [9-12]. Emerging evidence suggests that periodontitis and these chronic diseases share common risk factors and immunopathological mechanisms involving host-microbial dysbiosis, highlighting the potential for periodontal treatment to improve systemic health outcomes [12]. This intricate relationship between oral and systemic health has stimulated interest in exploring adjunctive therapies that extend benefits beyond local periodontal treatment [13].
Unlike antibiotics, probiotics restore microbial balance while suppressing the growth of pathogenic microbes in both the oral cavity and the gut. This makes them a promising adjunctive approach to periodontal treatment [14], particularly in the management of periodontal disease associated with systemic disease [15]. Sabatini et al.[16] demonstrated that probiotic supplementation in diabetic patients with gingivitis significantly improved both gingival health and glycemic control. In addition, consumption of probiotics produces short-chain fatty acids and bacteriocins, creating an unfavorable environment for pathogenic bacteria in the gut [17], and can reduce systemic inflammation through decreasing ROS and inflammatory metabolites [18]. These findings highlight the potential of probiotics to positively infuence both oral health and systemic health parameters.
Previous studies have primarily focused on evaluating the effects of individual probiotic strains or analyzing either periodontal or metabolic indices separately. However, studies that comprehensively analyze both periodontal and metabolic indices to assess whether multi-strain probiotics can influence systemic metabolic health remain limited. Therefore, this retrospective study aims to evaluate the clinical efficacy of multi-strain probiotics as an adjunct to NSPT, with a particular focus on their effects on chronic disease highly associated with periodontal disease, specifically DM and cardiovascular disease.
The study included a continuous cohort of outpatients at the Department of Oral and Maxillofacial Surgery at Apple Tree Dental Hospital between 1 June 2023 and 31 July 2024 who underwent non-pharmacological treatment after being diagnosed with chronic periodontitis and agreed to donate their biospecimens and the related clinical data to the Apple Tree Oral Biobank at the Apple Tree Medical Foundation, a part of Korea Biobank Network operated by Korea National Institute of Health. The study was approved by the Institutional Review Board of Apple Tree Medical Foundation (approval number: ATDH-2024-0007) and was conducted in accordance with the 1975 Declaration Helsinki (World Medical Association, revised in 2013).
Study participants underwent a comprehensive treatment regimen consisting of periodontal debridement, oral hygiene education, and daily administration of a probiotic supplement for 12 weeks <Fig. 1>. The use of other probiotic supplements was prohibited during the treatment. Clinical and laboratory assessments, including periodontal examination, analysis of oral pathogenic bacteria, blood pressure measurements, and blood chemistry tests, were performed at baseline and post intervention by trained clinicians according to standardized protocols.
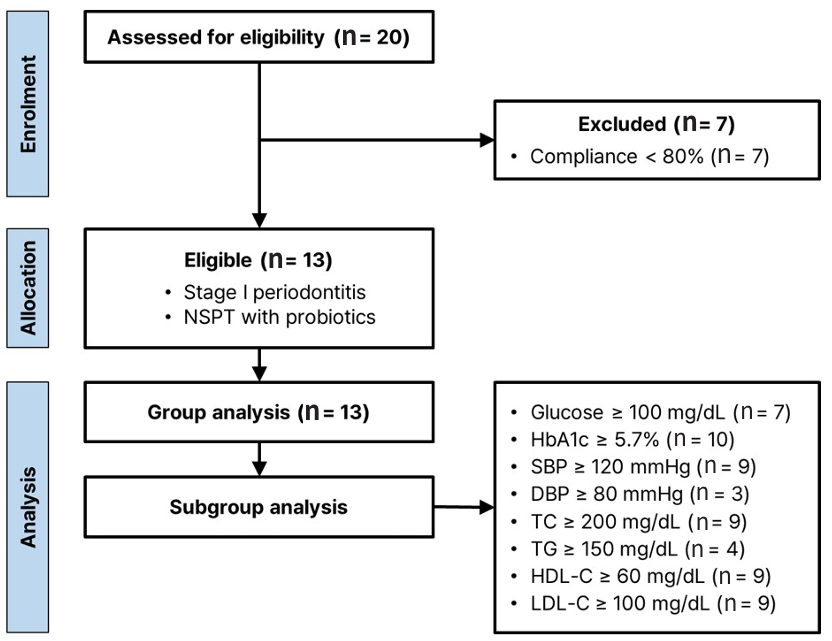
Fig. 1. Flowchart of the retrospective pilot study
The study included participants aged 19 to 79 years who had at least one of the following parameters above normal reference values: (i) FBG ≥100 mg/dL, (ii) glycated hemoglobin (HbA1c) ≥5.7%, (iii) systolic blood pressure (SBP) ≥120 mmHg, (iv) diastolic blood pressure (DBP) ≥80 mmHg, (v) total cholesterol (TC) ≥200 mg/dL, (vi) TG ≥150 mg/dL, (vii) HDL-C ≤60 mg/dL, and (viii) LDL-C ≥100 mg/dL. Patients were excluded if they had any serious systemic disease, including cardiovascular disease, immunological disorder, respiratory disease, gastrointestinal or biliary disease, neurological disorder, musculoskeletal disease, or malignant infectious disease. In addition patients with moderate to severe dental condition (e.g., periodontitis stage II-IV, caries, xerostomia, or peri-implantitis), uncontrolled hypertension (SBP/DBP ≥160/100 mmHg after 10 minutes of rest), or uncontrolled DM (FBG ≥180 mg/dL or initiation of DM medication within the previous three months) were excluded. Individuals with psychiatric disorders (e.g., schizophrenia, depression, substance abuse, or alcohol abuse) or a history of bleeding disorders, including current use of antiplatelet or anticoagulant medications, were also excluded. Women who were pregnant, planning to be pregnant, breastfeeding, or within six months of giving birth were not eligible to participation.
Data were retrieved from the electronic medical record system of Apple Tree Dental Hospital, including demographic information and clinical outcomes. All data were anonymized, securely stored as PDF files, and encrypted to ensure patient confidentiality, to authorised researchers for study purposes only.
The PPD and BOP indices were obtained by averaging the measurements at the mesiobuccal, mid-buccal, distobuccal, mesiolingual, mid-lingual, and distolingual sites of the Ramfjord teeth (maxillary right first molar, maxillary left central incisor, maxillary left first premolar, mandibular left first molar, mandibular right central incisor, and mandibular right first premolar) [19]. A North Carolina periodontal probe (Hu-Friedy, IL, USA) was used with all measurements rounded to the nearest millimeter.
The periodontal pathogens were analyzed by extracting genomic DNA from mouthwash samples using the Bacteria Genomic DNA Isolation Kit (LaboPassTM, Cosmogenetech, Korea), followed by quantitative PCR (qPCR) analysis of six bacterial species (Porpyromonas gingivalis, Tannerella forsythia, Treponema denticola, Prevotella intermedia, Fusobacterium nucleatum, and Streptococcus mutans) as previously reported [19].
Clinical diagnoses were performed by trained nurses and board-certified clinical pathologists. Blood pressure was measured using a calibrated sphygmomannometer after 5 minute of seate rest. Blood samples (4 mL) were collected by venupuncture into BD Vacutainer® SST™ tubes after an 8-hour fast. Samples were centrifuged at 3,000 rpm for 10 minutes using the DM0408 centrifuge (DLAB Scientific, China) to obtain serum. Biochemical analyses, including FBG, TC, TG, HDL-C, and LDL-C, were performed on an A25 automated analyzer (BioSystems, Spain). HbA1c levels were measured using the A1Care® analyzer (i-SENS, Korea) according to the manufacturer’s instructions. All biochemical parameters were measured in duplicate, and the mean values were used for statistical analyses.
Adherence to the probiotic regimen was assessed by verbal confirmation and the number of remaining probiotic sachets remaining at the time of the final visit (second visit). Participants who reported having three or fewer sachets remaining at the final visit were considered eligible for inclusion in the analysis.
The periodontal debridement included supra- and subgingival instrumentation at all sites using 11/12 and 13/14 Gracey curettes (Hu-Friedy, IL, USA) and ultrasonics (EMS, Switzerland). The oral health recommendations included brushing with a manual toothbrush for at least minimum of 3 min, twice a day, and using interdental brushes to clean between the teeth. Instructions were tailored to the patient’s specific needs for optimal plaque control. The patients were then instructed to take one sachet (3 g) of powdered probiotics (DOCSmedi, Korea) daily for 12 weeks. The composition of the probiotic product is detailed in <Table 1>.
Table 1. Composition of the probiotic supplement
| Probiotic regimen | Amount per sachet (3 g) |
|---|---|
| Lactobacillus strains (L. plantarum DM083, L. fermentum DM072, L. fermentum DM075, L. rhamnosus DM163) | 109 CFU |
| Vitamin D | 10 μg |
| Zinc | 8.5 mg |
| Selenium | 55 μg |
| Carbohydrates | 3 g |
The entire patient population was evaluated, with subgroups formed based on participants who exceeded normal reference values for disease-specific variables. Changes in these subgroup-defining variables were analyzed before and after the intervention. Data analysis was performed using R software (ver.4.4.2) with ggplot2 package (ver.3.5.1). Continuous variables were presented as mean and standard deviation (SD) or median with interquartile range (IQR), depending on the data distribution. Categorical variables were summarized as frequencies and percentages. Paired t-tests were used for normally distributed data, while the Wilcoxon signed-rank test was used for non-normally distributed data to assess changes in clinical outcomes after probiotic use. A p<0.05 was considered statistically significant for all analyses.
A total of 13 out of 20 patients undergoing non-pharmacological treatment were included in the study. The study population comprised 11 women and 2 men, ranging in age from 43 to 68 years, with a mean age of 55.6 (±8.5) years. Of the enrolled participants, 15.4% were smokers. Participants attended the clinic a minimum of 2 times and a maximum of 3 times, with a mean of 2.46 visits. Follow-up time ranged from a minimum of 85 days to a maximum of 140 days, with an average of 107.7 days. Importantly, none of patients enrolled reported any treatment-emergent adverse events.
Twelve weeks of NSPT with concomitant probiotic supplementation did not result in statistically significant improvements in PPD, the maximum PPD (PPDmax ), or BOP <Table 2>; however, periodontal indices remained stable throughout the intervention period. However, blood tests showed a statistically significant reduction in HbA1c levels (0.25, 4.28%, p<0.01). In addition qPCR analysis of six oral pathogenic bacteria showed a significant reduction in T. forsythia levels (p<0.05) <Fig. 2>. Although statistically significant, reductions in other bacterial species, including P. gingivalis, P. intermedia, F. nucleatum, and S. mutans were also observed.
Table 2. Clinical outcomes after 12 weeks of NSPT with probiotic supplementation
(N=13)
| Variables | Baseline | 12 weeks | p |
|---|---|---|---|
| PPD (mm) | 2.08±0.36 | 2.02±0.32 | 0.588* |
| PPDmax (mm) | 2.82±0.51 | 2.71±0.42 | 0.467* |
| BOP (%) | 12.52±11.99 | 16.11±12.36 | 0.447* |
| Glucose | 95.15±12.52 | 97.85±10.85 | 0.724** |
| HbA1c (%) | 5.84±0.37 | 5.59±0.34 | 0.001* |
| SBP (mmHg) | 124.23±13.01 | 118.38±12.75 | 0.139* |
| DBP (mmHg) | 74.54±7.37 | 70.85±11.63 | 0.228* |
| TC (mg/dL) | 227.08±41.94 | 234.08±49.60 | 0.354* |
| TG (mg/dL) | 115.46±56.58 | 127.08±78.13 | 0.517* |
| HDL-C (mg/dL) | 63.38±10.46 | 65.28±14.35 | 0.372* |
| LDL-C (mg/dL) | 120.85±30.97 | 118.00±33.18 | 0.622* |
*by paired t-test, **by Wilcoxon signed-rank test
Values are presented as Mean±SD.
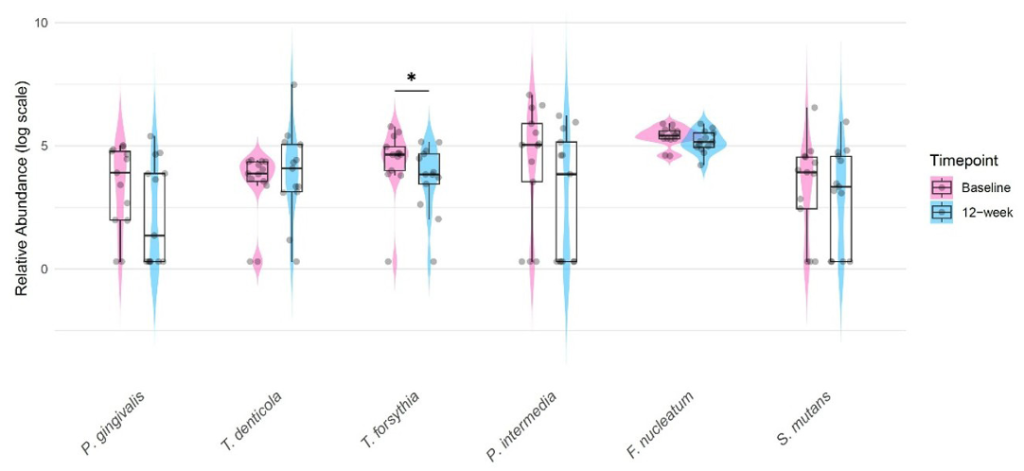
Fig. 2. Changes in oral pathogenic bacteria after 12 weeks of probiotic use as an adjunct to periodontal debridement in all patients (N=13). Superimposed violin, dot, and box plots with IQR were generated using R with the ggplot2 package. *p<0.05 by Wilcoxon signed-rank test on the raw data
Subgroups were categorized on the basis of the normal reference values for blood test results. <Table 3> shows the demographic characteristics of these subgroups and the changes in the intervention outcomes. The HbA1c subgroup was the largest with ten participants, while the DBP subgroup was the smallest, with only three participants. The TG subgroup had the highest mean age, whereas the DBP subgroup had the lowest.
Analysis of metabolic syndrome-related variables after the intervention showed significant improvements in the HbA1c and SBP subgroups. In the HbA1c subgroup (N=10, HbA1c≥5.7%), mean HbA1c decreased by 0.3 (5%, p<0.001). In the SBP subgroup (N=9, SBP≥120 mmHg), mean SBP decreased by 9.6 (7.3%, p<0.01). No significant changes were observed in the other subgroups.
Table 3. Subgroup analysis according to the groups defined by baseline thresholds
| Subgroup | N | Age | Sex (female) | Smoker | Intervention | p* | |||
|---|---|---|---|---|---|---|---|---|---|
| N(%) | N(%) | Baseline | 12 weeks | ||||||
| Glucose≥100 mg/dL | 7 | 56.1±8.6 | 7(100.0) | 1(14.3) | 103.4±3.0 | 96.1±8.1 | 0.060 | ||
| HbA1c≥5.7% | 10 | 52.7±7.4 | 10(100.0) | 2(20.0) | 6.0±0.2 | 5.7±0.2 | <0.001 | ||
| SBP≥120 mmHg | 9 | 54.8±8.9 | 8(88.9) | 2(22.2) | 131.3±7.3 | 121.7±10.7 | 0.007 | ||
| DBP≥80 mmHg | 3 | 51.7±14.2 | 3(100.0) | 0(0.0) | 84.3±4.0 | 73.3±19.3 | 0.381 | ||
| TC≥200 mg/dL | 9 | 56.0±8.8 | 8(88.9) | 2(22.2) | 248.3±28.8 | 256.0±42.1 | 0.489 | ||
| TG≥150 mg/dL | 4 | 59.5±7.9 | 3(75.0) | 1(25.0) | 185.0±18.3 | 183.0±63.0 | 0.961 | ||
| HDL-C≥60 mg/dL | 9 | 55.1±9.2 | 7(77.8) | 1(11.1) | 68.2±8.4 | 71.8±10.0 | 0.122 | ||
| LDL-C≥100 mg/dL | 9 | 56.6±8.4 | 8(88.9) | 2(22.2) | 137.7±18.6 | 129.8±30.0 | 0.268 | ||
*by paired t-test, Values are presented as Mean±SD.
This retrospective study aimed to evaluate the clinical efficacy of NSPT combined with multi-strain probiotic supplementation and comprehensively analyze both periodontal and metabolic indices to explore its impact on systemic health. Current periodontal disease models emphasize the importance of host-microbial homeostasis, suggesting that adjunctive probiotic therapy with NSPT may aid in restoring microbial balance and positively influence systemic immune regulation. Unlike previous studies that primarily focused on improving periodontal health, this study sought to integrate the assessment of both periodontal and metabolic health to better clarify the effects of multi-strain probiotics on both oral and systemic health.
The minimal changes observed in periodontal indices might be attributed to both the relatively mild baseline periodontitis severity and the complexity of host-microbial interactions in metabolic syndrome patients, which corresponds to stage I periodontitis [21]. In this study, only patients with Stage I periodontitis or mild periodontal conditions were included to evaluate the effects of early intervention with probiotics in the initial stage. A recent systematic review shown that a greater reduction in PPD is associated with more substantial mean PPD reduction [22]. However, the lack of improvement in mean BOP suggests that NSPT combined with probiotic therapy was ineffective in reducing gingival inflammation within the 12-week intervention period. The chronic nature of periodontal tissue destruction and the complex inflammatory processes involved may require longer intervention periods to reverse these established clinical manifestations of periodontal disease with the therapeutic regimen used in this study. Future studies should consider evaluating the inflammatory cytokine profiles to better understand the immunomodulatory effects of probiotic supplementation.
Although periodontal indices such as PPD and BOP remained stable without significant improvements, the stability of these parameters over 12 weeks suggests that adjunctive probiotic therapy may have helped maintain periodontal health during NSPT, preventing further deterioration. Microbiological analysis showed a significant reduction in T. forsythia, while all six bacterial species showed a decreasing trend following probiotic supplementation. T. forsythia is a key periodontopathogen associated with disease progression, and its reduction has been linked to improved periodontal outcomes. The significant reduction in T. forsythia levels in consistent with previous findings by Cho et al. [23] and Pudgar et al. [24] and may be due to the nature of Lactobacillus strains that can inhibit T. forsythia through competitive exclusion and the production of specific bacteriocin [25]. These findings suggest that probiotic supplementation may have played a role in suppressing periodontal pathogens, potentially contributing to microbial homeostasis in the oral cavity.
Recent systematic reviews and meta-analyses have shown that NSPT without any additional therapy significantly reduced HbA1c levels in periodontitis patients with DM (0.16) and without DM (0.31) at 12 weeks follow-up [26,27]. A recent systematic review metaanalyzed 21 randomized controlled trials and showed a significant reduction in SBP and other cardiovascular disease risk markers after NSPT [28]. This finding is clinically meaningful as it suggests that improvements in key metabolic health markers, such as blood glucose and blood pressure, can be achieved through non-pharmacological interventions, including periodontal care and probiotic supplementation. The observed metabolic improvements may be attributed to the ability of probiotics to enhance insulin sensitivity, modulate gut microbiota composition, and reduce systemic inflammation by suppressing pro-inflammatory cytokines such as TNF-α and IL-6 [29].
The oral cavity strain L. fermentum DM075 can reduce nitrate (NO3-) to nitrite (NO2-) [30] and may participate in the human nitrate-nitrite-nitric oxide pathway to promote vasodilation and improve endothelial function [31]. The strain L. fermentum DM072, also isolated from the oral cavity, can produce hydrogen peroxide and suppress the growth of S. mutans on the tooth surface [32]. In addition, an in vivo study using L. plantarum DM083 has been shown to reduce postprandial glucose levels, while L. rhamnosus DM163 has anti-inflammatory functions in vitro (both data to be published elsewhere). These findings suggest that the combination of different Lactobacillus strains with multiple functions in our probiotic supplement may provide complementary mechanisms for improving cardiovascular health, particularly in individuals with elevated blood pressure. However, the promising in vitro and in vivo data should be further evaluated in clinical trials.
The absence of significant changes in lipid profiles may be attributed to the strain-specific effects of probiotics, where some strains impact glucose metabolism and blood pressure regulation more than lipid metabolism. Some previous findings, such as the case report, showed improvements in cholesterol parameters with probiotic supplementation [23]. This discrepancy may be due to several factors, including the strain-specific effects of probiotics, with some strains appearing to affect glucose metabolism and blood pressure regulation more than lipid metabolism. Previous studies have suggested that while certain probiotic strains may affect lipid profiles through mechanisms such as bile salt hydrolase activity and cholesterol assimilation, results may require longer treatment durations or higher doses to become apparent [33]. Furthermore, the relatively short intervention period of 12 weeks may not have been sufficient to observe significant changes in lipid metabolism, suggesting the need for longer observation periods in future studies focusing on dyslipidemia.
This study was conducted in accordance with routine clinical protocols. As a result, research-specific parameters such as clinical attachment loss were not included due to practical limitations in data collection. The limitations of the study, particularly the small sample size and the recruitment of participants from a single hospital, should be noted, as they may limit the generalizability of the findings and reduce the statistical power to detect significant changes in all measured parameters. In addition, the study included a broad age range (19-79 years), which may have introduced variability in general health status and oral conditions. Given the small sample size, subgroup analyses, particularly in groups with very few participants (e.g., DBP subgroup, n=3), should be interpreted with caution due to the increased risk of statistical bias. Moreover, the absence of a control group limits the ability to establish a causal relationship between the intervention and observed effects. The lack of multivariate analysis further restricts the ability to account for potential confounding factors. Additionally, as this study was of relatively short duration, long-term follow-up studies are needed to determine the sustained effects of probiotics on periodontal and systemic health. To address this, inclusion criteria were carefully defined, and efforts were made to design a multicenter study to capture a more diverse population. Larger-scale, randomized controlled trials are needed to confirm these results and to explore the full range of benefits that multi-strain probiotics can offer in both periodontal and systemic health management. Furthermore, optimizing the combination of strains, dosage, and duration of treatment is crucial for maximise the therapeutic potential of probiotics in different patient populations.
This retrospective study offers valuable insights into emerging periodontal approaches utilizing probiotic supplementation for both oral microbial modulation and metabolic improvement.
1. Probiotic supplementation with NSPT may improve glycemic control and blood pressure regulation
2. Limited effects on periodontal clinical parameters observed
3. Significant potential for managing metabolic co-morbidities in periodontal patients
This highlights the promising link between periodontal treatment approaches and systemic health outcomes.
Conceptualization: MY Cho, HS Kim, IS Hwang; Data collection: MY Cho, JH Eom, EM Choi; Formal analysis: MY Cho, JY Hwang, IS Hwang; Writing-original draft: MY Cho, IS Hwang; Writing-review&editing: MY Cho, JY Hwang, JH Eom, EM choi, JH Lee, YJ Kim, JY Hwang, HS Kim, IS Hwang
H.-S.K holds stocks of DOCSmedi, Co., Ltd. The remaining authors have no conflict of interest to disclose.
This research was supported in part by the Korea National Institute of Health (KNIH) research project (No. 2024ER050700).
This study was approved by the Institutional Review Board (IRB) of Institutional Review Board of Apple Tree Medical Foundation (IRB No. ATDH-2024-0007).
Data can be obtained from the corresponding author.
We used ChatGPT 4o to correct grammatical errors of the manuscript.
1. Neeland IJ, Lim S, Tchernof A, Gastaldelli A, Rangaswami J, Ndumele CE, et al. Metabolic syndrome. Nat Rev Dis Primers 2024;10(1):77. https://doi.org/10.1038/s41572-024-00563-5
[DOI][PubMed]
2. Ross FC, Patangia D, Grimaud G, Lavelle A, Dempsey EM, Ross RP, et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol 2024;22(11):671-86. https://doi.org/10.1038/s41579-024-01068-4
[DOI][PubMed]
3. Kunath BJ, De Rudder C, Laczny CC, Letellier E, Wilmes P. The oral-gut microbiome axis in health and disease. Nat Rev Microbiol 2024;22(12):791-805. https://doi.org/10.1038/s41579-024-01075-5
[DOI][PubMed]
4. de Oliveira AM, Lourenço TGB, Colombo APV. Impact of systemic probiotics as adjuncts to subgingival instrumentation on the oral-gut microbiota associated with periodontitis: a randomized controlled clinical trial. J Periodontol 2022;93(1):31-44. https://doi.org/10.1002/JPER.21-0078
[DOI][PubMed]
5. Shah NP. Functional cultures and health benefits. Int Dairy J 2007;17(11):1262-77. https://doi.org/10.1016/j.idairyj.2007.01.014
[DOI]
6. Chen T, Wang J, Liu Z, Gao F. Effect of supplementation with probiotics or synbiotics on cardiovascular risk factors in patients with metabolic syndrome: a systematic review and meta-analysis of randomized clinical trials. Front Endocrinol (Lausanne) 2024:14:1282699. https://doi.org/10.3389/fendo.2023.1282699
[DOI][PubMed][PMC]
7. Su Y, Cao Y, Bing X, Chen C, Yan H, Zhao H, et al. Effect of non-surgical periodontal therapy on coronary artery disease or metabolic syndrome: a systematic review and meta-analysis. J Evid Based Dent Pract 2025:102136. https://doi.org/10.1016/j.jebdp.2025.102136
[DOI]
8. Trindade D, Carvalho R, Machado V, Chambrone L, Mendes JJ, Botelho J. Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta-analysis of epidemiological studies. J Clin Periodontol 2023;50(5):604-26. https://doi.org/10.1111/jcpe.13769
[DOI][PubMed]
9. Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000 2020;83(1):7-13. https://doi.org/10.1111/prd.12344
[DOI][PubMed]
10. Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol 2021;21(7):426-40. https://doi.org/10.1038/s41577-020-00488-6
[DOI][PubMed][PMC]
11. Villoria GEM, Fischer RG, Tinoco EMB, Meyle J, Loos BG. Periodontal disease: a systemic condition. Periodontol 2000 2024;96(1):7-19. https://doi.org/10.1111/prd.12616
[DOI][PubMed][PMC]
12. Herrera D, Sanz M, Shapira L, Brotons C, Chapple I, Frese T, et al. Periodontal diseases and cardiovascular diseases, diabetes, and respiratory diseases: summary of the consensus report by the European Federation of Periodontology and WONCA Europe. Eur J Gen Pract 2024;30(1):2320120. https://doi.org/10.1080/13814788.2024.2320120
[DOI][PubMed][PMC]
13. Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc 2015;146(7):525-35. https://doi.org/10.1016/j.adaj.2015.01.026
[DOI][PubMed]
14. Puzhankara L, Banerjee A, Chopra A, Venkitachalam R, Kedlaya MN. Effectiveness of probiotics compared to antibiotics to treat periodontal disease: systematic review. Oral Dis 2024;30(5):2820-37. https://doi.org/10.1111/odi.14781
[DOI][PubMed]
15. de Brito Avelino L, Rodrigues KT, da Silva Cruz NT, Martins AA, de Aquino Martins ARL. Effectiveness of probiotic therapy in the management of periodontal disease in diabetic patients: a scoping review. Curr Diabetes Rev 2024;20(9):e281123223961. https://doi.org/10.2174/0115733998271193231108054254
[DOI][PubMed]
16. Sabatini S, Lauritano D, Candotto V, Silvestre FJ, Nardi GM. Oral probiotics in the management of gingivitis in diabetic patients: a double blinded randomized controlled study. J Biol Regul Homeost Agents 2017;31(2):197-202.
17. Anjana, Tiwari SK. Bacteriocin-producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Front Cell Infect Microbiol 2022;12:851140. https://doi.org/10.3389/fcimb.2022.851140
[DOI][PubMed][PMC]
18. Colletti A, Pellizzato M, Cicero AF. The possible role of probiotic supplementation in inflammation: a narrative review. Microorganisms 2023;11(9):2160. https://doi.org/10.3390/microorganisms11092160
[DOI][PubMed][PMC]
19. Ramfjord SP. Indices for prevalence and incidence of periodontal disease. The Journal of Periodontology 1959;30(1):51-9. https://doi.org/10.1902/jop.1959.30.1.51
[DOI]
20. Hwang JY, Lee JH, Kim YJ, Hwang IS, Kim YY, Kim HS, et al. Highly accurate measurement of the relative abundance of oral pathogenic bacteria using colony-forming unit-based qPCR. J Periodontal Implant Sci 2024;54(6):444-57. https://doi.org/10.5051/jpis.2304520226
[DOI][PubMed][PMC]
21. Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions – Introduction and key changes from the 1999 classification. J Periodontol 2018;89(S1):S1-S8. https://doi.org/10.1002/JPER.18-0157
[DOI][PubMed]
22. Suvan J, Leira Y, Moreno Sancho FM, Graziani F, Derks J, Tomasi C. Subgingival instrumentation for treatment of periodontitis. a systematic review. J Clin Periodontol 2020;47(S22):155-75. https://doi.org/10.1111/jcpe.13245
[DOI][PubMed]
23. Cho MY, Hwang IS, Kim YY, Kim HS. Changes in periodontal pathogens and chronic disease indicators through adjunctive probiotic supplementation: a case report. J Korean Soc Dent Hyg 2024;24(2):91-8. https://doi.org/10.13065/jksdh.20240010
[DOI]
24. Pudgar P, Povšič K, Čuk K, Seme K, Petelin M, Gašperšič R. Probiotic strains of Lactobacillus brevis and Lactobacillus plantarum as adjunct to non-surgical periodontal therapy: 3-month results of a randomized controlled clinical trial. Clin Oral Investig 2021;25(3):1411-22. https://doi.org/10.1007/s00784-020-03449-4
[DOI][PubMed]
25. Kang MS, Oh JS, Lee HC, Lim HS, Lee SW, Yang KH, et al. Inhibitory effect of Lactobacillus reuteri on periodontopathic and cariogenic bacteria. J Microbiol 2011;49(2):193-9. https://doi.org/10.1007/s12275-011-0252-9
[DOI][PubMed]
26. Sun Y, Zhang W, Lu L, Zhao D, Wang S, Pan Y, et al. Effect of non-surgical periodontal therapy on hemoglobin A1c in periodontitis patients without diabetes mellitus: a systematic review and meta-analysis. J Dent 2024;145:104974. https://doi.org/10.1016/j.jdent.2024.104974
[DOI][PubMed]
27. Corbella S, Alberti A, Donos N, Morandi B, Ercal P, Francetti L, et al. Efficacy of different protocols of non-surgical periodontal therapy in patients with type 2 diabetes: a systematic review and meta-analysis. J Periodontal Res 2024. https://doi.org/10.1111/jre.13327
[DOI][PubMed]
28. Meng R, Xu J, Fan C, Liao H, Wu Z, Zeng Q. Effect of non-surgical periodontal therapy on risk markers of cardiovascular disease: a systematic review and meta-analysis. BMC Oral Health 2024;24(1):692. https://doi.org/10.1186/s12903-024-04433-0
[DOI][PubMed][PMC]
29. Yarahmadi M, Javid AZ, Bazyar H, Yousefimanesh HA, Nejatian T, Gravand E, et al. The effects of synbiotic supplementation along with non-surgical periodontal therapy in improving the metabolic status and inflammatory markers in type 2 diabetes mellitus patients with periodontal disease: a double-blind randomized clinical trial. J Edu Health Promot 2024;13(1):430. https://doi.org/10.4103/jehp.jehp_1382_23
[DOI]
30. Park DY, Hwang JY, Kim YJ, Kim YY, Kim HS, Hwang IS. Whole-Genome Sequence of Limosilactobacillus fermentum Strain DM075, Isolated from the Human Oral Cavity. Microbiol Resour Announc 2022;11(11):e0081922. https://doi.org/10.1128/mra.00819-22
[DOI][PubMed][PMC]
31. Chai X, Liu L, Chen F. Oral nitrate-reducing bacteria as potential probiotics for blood pressure homeostasis. Front Cardiovasc Med 2024;11:1337281. https://doi.org/10.3389/fcvm.2024.1337281
[DOI][PubMed][PMC]
32. Park DY, Hwang JY, Kim YJ, Lee DH, Kim YY, Kim HS, et al. Antimicrobial activity of Limosilactobacillus fermentum strains isolated from the human oral cavity against Streptococcus mutans. Sci Rep 2023;13(1):7969. https://doi.org/10.1038/s41598-023-35168-7
[DOI][PubMed][PMC]
33. Wu Y, Zhang Q, Ren Y, Ruan Z. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PloS One 2017;12(6):e0178868. https://doi.org/10.1371/journal.pone.0178868
[DOI][PubMed][PMC]