Introduction
Osteoporosis, a prevalent bone disorder that predominantly affecting the elderly, is characterized by reduced bone density and increased fractures susceptibility [1]. At the cellular level, it arises from the dysregulation of osteoclast activation-a pivotal process in maintaining the balance between bone formation and resorption [2]. Despite pharmaceutical advances in the treatment of osteoporosis, synthetic drugs often have undesirable side effects that affect patients of well-being. Gastrointestinal discomfort, skin reactions, and muscle pain, along with an increased risk of fracture highlight the need for safer alternatives [3]. This has led to research into natural remedies with minimal side effects for the treatment of patients with osteoporosis.
Recent studies have demonstrated the promising properties (e.g., anti-inflammatory and antioxidant activities) of rhubarb extract, which is derived from a traditional medicinal plant [4]. Osteoclasts are crucial for bone resorption and differentiate from hematopoietic stem cells into multinucleated cells (MNCs) through the fusion of pre-osteoclasts/monocytes [5]. Osteoclast differentiation is initiated by the receptor activator of NF-κB ligand (RANKL) through a process involving key transcription factors such as NF-κB, c-Fos, and NFATc1 [2].
This study investigated the effect of rhubarb extract on osteoclast differentiation, a central process of bone resorption. Osteoclast activity plays an important role in a variety of oral health problems, and dysregulation of osteoclasts can directly affect oral health, including conditions such as periodontal disease and tooth loss. he investigation of natural extracts like rhubarb reflects the quest for treatments that are minimally invasive and safe, holding potential implications for overall oral health beyond just bone health.
Methods
1. Preparation of rhubarb extract (rhubarb E)
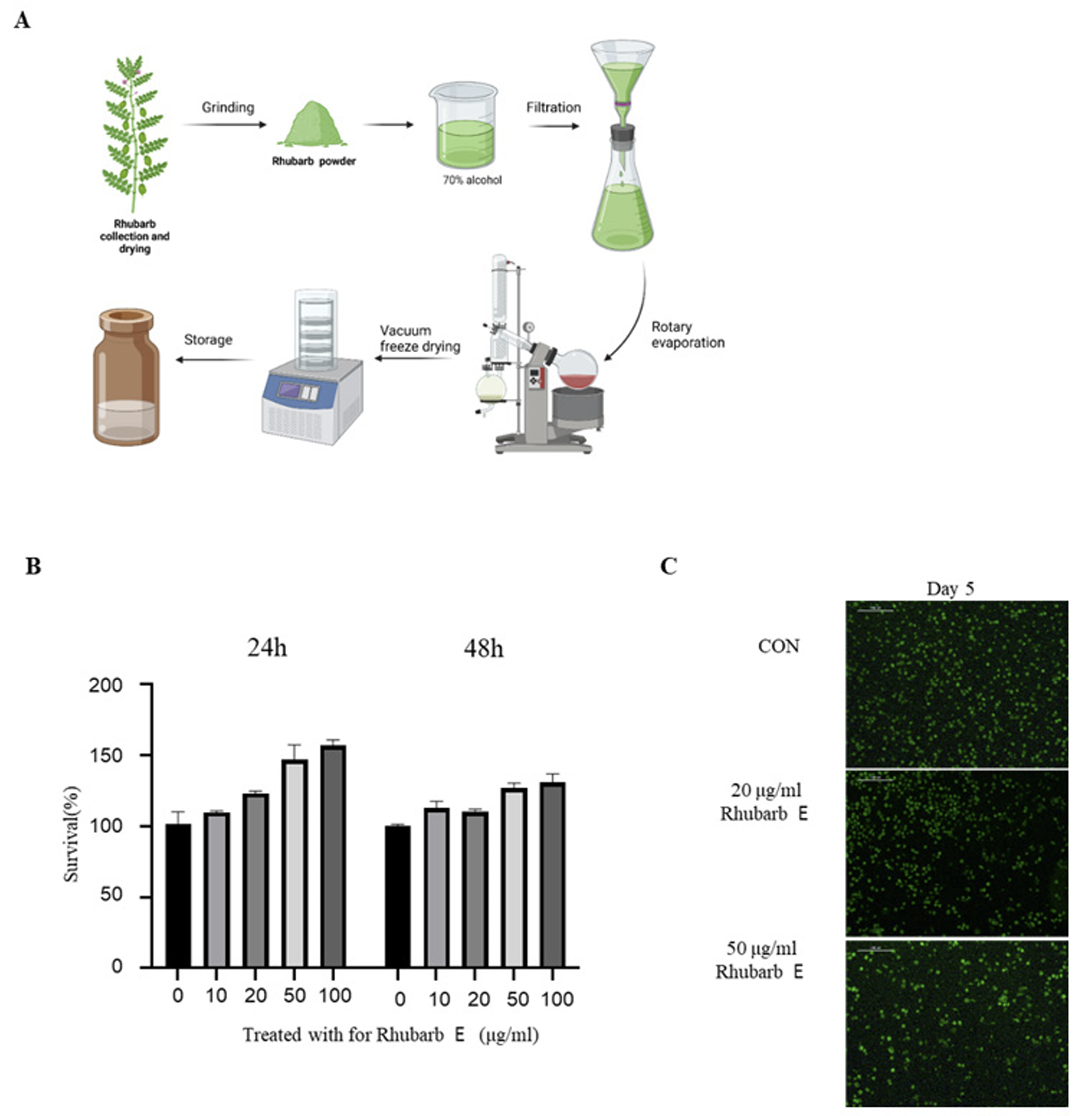
The procedure for the preparation of Rhubarb E is shown in (Fig. 1A). Briefly, dried rhubarb (Rheum palmatum) purchased from the herbal medicine market in Gwangju was finely ground using a grinder and blended with 70% alcohol at a ratio of 1:10. The resulting mixture was kept at room temperature for 3 days, and the obtained extract was filtered twice using a Whatman No. 2 filter paper (Whatman International Ltd.). The filtrate was concentrated under reduced pressure then lyophilized to obtain powdered Rhubarb E, which was further dissolved in dimethyl sulfoxide (DMSO; VWR Life Science, Solon, OH, USA) to obtain a concentration of 50 mg/mL for experimental use.
Fig. 1
Preparation of rhubarb extract (Rhubarb E) and the effect of Rhubarb E on the viability of BMMs: (A) Schematic representation of the process of manufacturing Rhubarb E; (B) Cytotoxicity evaluation of Rhubarb E on BMMs. BMMs were exposed to various concentrations of Rhubarb E (10, 20, 50, and 100 μg/mL) for 24 and 48 hours. The viability of the BMMs was determined using the MTT assay. Data are presented as Mean±SD (n=3). Significance levels are inficated as *p≤0.05, **p ≤0.01; (C) Fluorescence microscopy-based visualization of live (green fluorescence) and dead or damaged (red fluorescence) BMMs after their treatment with Rhubarb E (20 and 50 μg/mL) for 5 days. Representative images are displayed.
2. Isolation of BMMs and in vitro osteoclast differentiation assay
To isolate primary bone marrow-derived macrophages (BMMs), the femur and tibia of 5 week old male C57BL/6 mice were obtained from Damool Science (Daejeon, Korea) were separated. Both ends of the tibia and femur were cut, and BMMs were collected from the intramedullary cavity using a 1 mL syringe. The collected BMMs were cultured for 3 days in MEM Alpha (Gibco, Carlsbad, CA, USA) rowth medium containing 10% FBS (FBS, 10%, Gibco, Carlsbad, CA, USA), 1% penicillin/streptomycin (Gibco, Carlsbad, CA, USA), and 30 ng/mL mouse macrophage colony-stimulating factor (M-CSF, 100 ng/ml, BioLegend, USA). Subsequently, the attached BMMs were treated with various concentrations of Rhubarb E in the presence of RANKL (100 ng/mL) to induce differentiation. The culture medium was replaced every other day.
3. Cytotoxicity assay
The cytotoxicity of Rhubarb E was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. BMMs were seeded in 96-well plates and exposed to different concentrations of Rhubarb E for 24 and 48 hours. Subsequently, the MTT reagent was added and the plates were incubated for 4 hours. The formazan crystals formed were solubilized with DMSO and the absorbance of the samples was measured at 565 nm absorbance using a microplate reader (Multiskan SkyHigh Microplate Reader, Thermo Fisher, USA). The viability of the BMMs was determined from the absorbance values recorded.
4. Live/dead cell imaging assay
To assess the effect of Rhubarb E on the viability of BMMs, a live/dead cell imaging assay was employed. BMMs were treated with different concentrations of Rhubarb E (20 and 50 μg/mL) and cultured for 5 days. After the incubation period, the cells were stained with a fluorescent dye to between live cells (evidenced by green fluorescence) and distingush dead or compromised cells (indicated by red fluorescence). The stained cells were visualized using a fluorescence microscope imaging system (DMIL LED/DFC450C, Leica, Germany).
5. Tartrate-resistant acid phosphatase (TRAP) staining
BMMs seeded in 24-well plates (2×104 cells/well) were treated with different concentrations of Rhubarb E (10, 20 and 50 μg/mL) and cultured for 5 days in the presence of 30 ng/mL M-CSF and 100 ng/mL RANKL. The medium was changed every 2 days. The plates were washed three times with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 20 min, and then stained with a staining solution (0.12 M sodium acetate, 50 mM sodium tartrate, LB fast red violet salt, and naphthol AS-MX phosphate, pH 5.2). Subsequently, the plates were washed with PBS and air-dried. Finally, the TRAP-positive MNCs with three or more nuclei were counted under a quantified using Image J software (Media Cybernetics, Inc., Rockville, MD, USA).
6. Quantitative real-time polymerase chain reaction (qPCR)
Total RNA extraction from BMMs was performed using the Cell&Tissue RNA Extraction Kit (Infusion Tech, Gyeonggi-do, Korea). The obtained total RNA was used for cDNA synthesis using SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was performed using Power SYBR Green PCR Master Mix (Life Technologies Ltd., Woolston Warrington, UK) and a StepOnePlus RealTime PCR System (Applied Biosystems, Foster City, CA, USA). Target gene expression was quantified by the comparative CT method using StepOne Software ver. 2.10 (Applied Biosystems, Waltham, MA, USA). GAPDH was employed as an internal control. The primer sequences used for qPCR are shown in (Table 1).
7. Filamentous (F)-actin ring formation
F-actin ring formation was evaluated to determine the extent of osteoclast maturation. BMMs were plated a 12-well plate at a density of 1×105 cells/well and treated with different concentrations of Rhubarb E (10, 20, and 50 μg/mL) or left untreated. They were stimulated with 100 ng/mL RANKL for 5 days. The BMMs were washed, fixed, and stained with rhodamine-phalloidin. Then, the F-actin ring Actin-StainTM Fluorescent Phalloidins (Red, Thermo Fisher, USA) formation was visualized using a fluorescence microscope (DMIL LED/DFC450C; Leica, Germany), and the size and number of the F-actin rings formed were calculated.
8. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using the GraphPad Prism 9 software (GraphPad Software Inc, San Diego, CA, USA). Results are presented as Mean±SD. Significance levels are inficated as *P≤0.05, ** P≤0.01.
Results
1. Rhubarb E does not affect the viability of BMMs
To directly assess the effect of Rhubarb E on the viability of osteoclasts, the cytotoxicity of Rhubarb E on BMMs was examined. As shown in (Fig. 1B), Rhubarb E showed no apparent cytotoxicity at the concentrations used in this study (10, 20, 50, and 100 μg/ mL). A live/dead assay further demonstrated that Rhubarb E did not affect the viability of BMMs at the concentrations used (Fig. 1C). These results suggested that the inhibitory effects of Rhubarb E were not related to its cytotoxic potential. Subsequent experiments to study osteoclast differentiation were based on these viability results.
2. Rhubarb E inhibits RANKL-induced formation of TRAP-Positive multinucleated osteoclasts
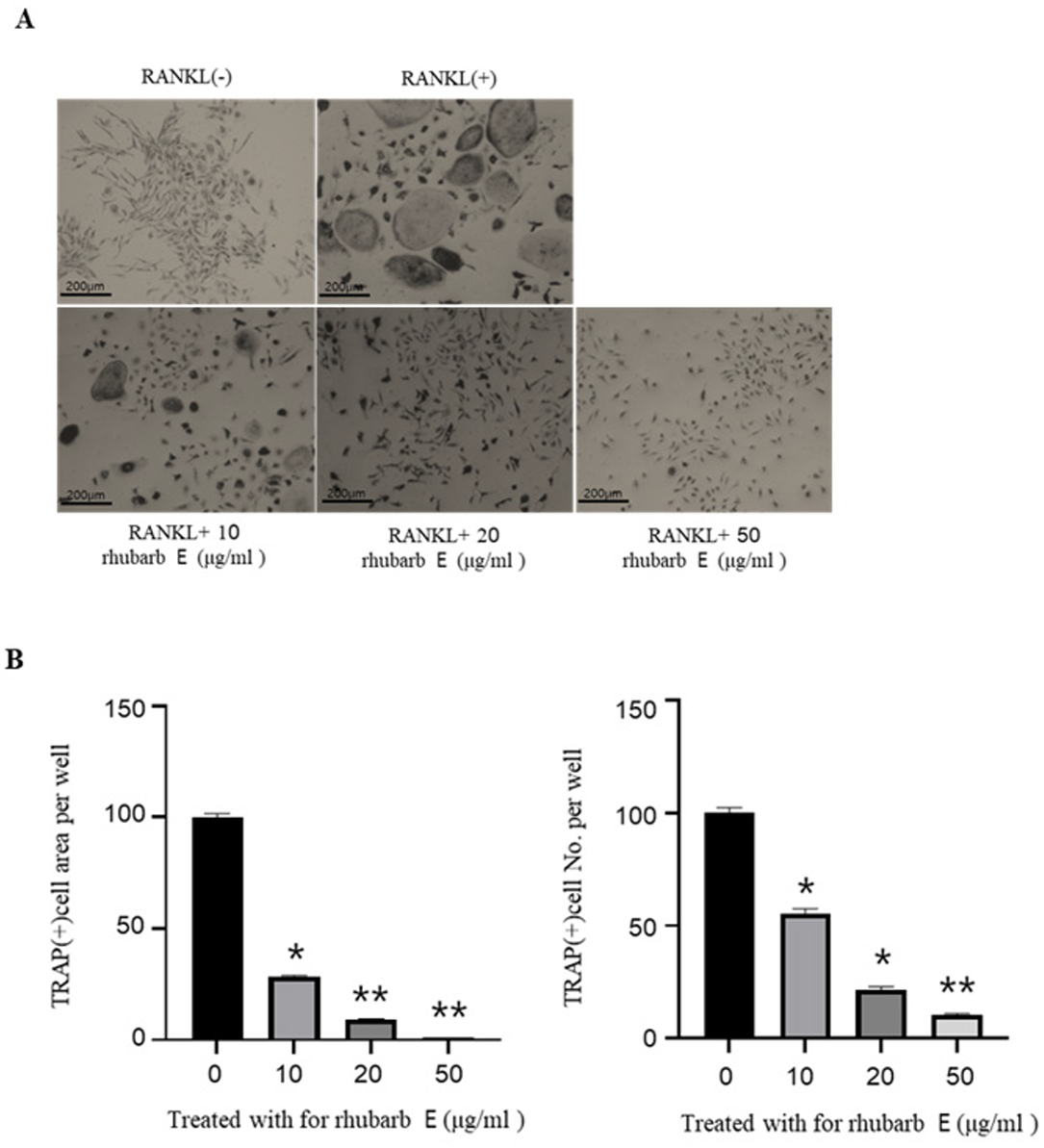
For TRAP staining, BMMs were cultured with Rhubarb E at concentrations of 10, 20, and 50 μg/mL in the presence of 30 ng/mL M-CSF and 100 ng/mL RANKL for 5 days. interestingly, Rhubarb E significantly suppressed the number of TRAP-positive MNCs in the BMM cultures in a concentration-dependent manner (Fig. 2A, 2C). Among the studied concentrations, the most robust inhibitory effect on RANK-induced TRAP positivity was observed at 50 μg/mL of Rhubarb E.
Fig. 2
Inhibition of osteoclast differentiation by Rhubarb E. (A) TRAP staining of BMMs treated with different concentrations of Rhubarb E (10, 20, and 50 μg/mL) and stimulated with 100 ng/mL RANKL for 5 days. TRAP-positive multinucleated cells (MNCs) are stained in red: (B) Quantitative analysis of TRAP-positive MNC area and cell number. Data were analyzed by oneway ANOVA followed by Tukey’s honest test and are presented as Mean±SD (n=3). Significance levels are inficated as *P≤ 0.05, **P≤0.01.
3. Rhubarb E downregulates osteoclast-specific gene expression
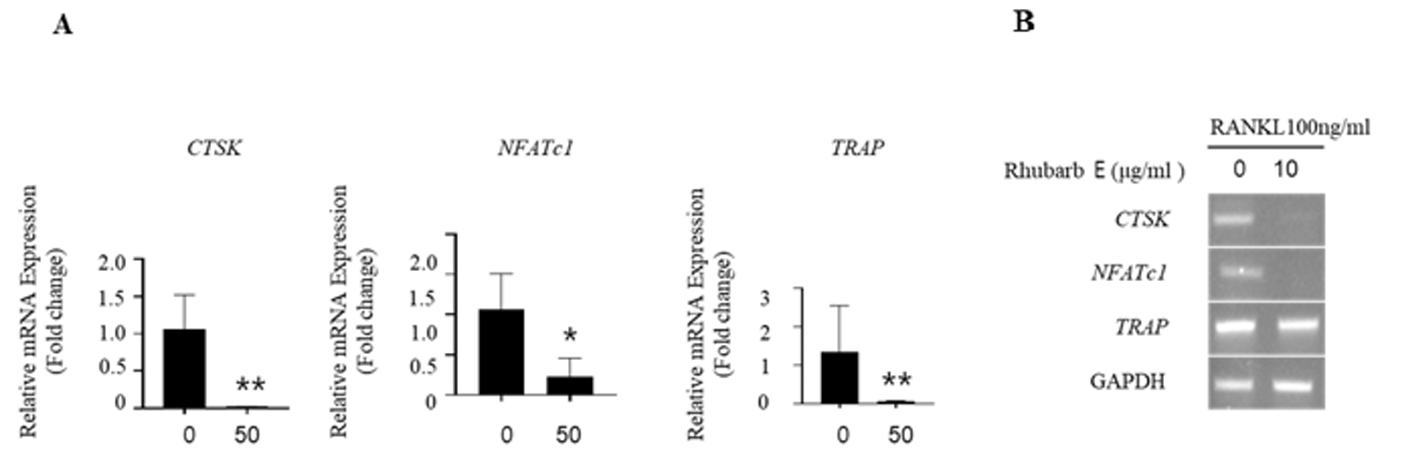
To validate the inhibitory effect of Rhubarb E on osteoclastogenesis, mRNA expression of the treated BMMs was analyzed. The expression of osteoclast-specific genes, such as cathepsin K, NFATc1, and TRAP, showed a significantly increase in the RANKLtreated group. However, the expression of these genes was significantly suppressed during osteoclastogenesis in the presence of Rhubarb E (Fig. 3A, 3B). This downregulation of critical genes associated with osteoclastogenesis underscores the inhibitory role of Rhubarb E on osteoclast differentiation.
Fig. 3
Downregulation of osteoclast-specific gene expression by Rhubarb E: (A) Analysis of mRNA expression of cathepsin K, NFATc1, and TRAP in BMMs treated with Rhubarb E (50 μg/mL) and stimulated with 100 ng/mL RANKL. Gene expression was quantified by qPCR and normalized to GAPDH. Data were analyzed using one-way ANOVA followed by Tukey’s honest test and are presented as Mean±SD (n=3). Significance levels are inficated as *P≤0.05, **P≤0.01; (B) Osteoclast-specific gene expression analysis by conventional qPCR.
4. Rhubarb E inhibits RANKL-induced F-actin ring formation
To further confirm the inhibitory effect of Rhubarb E on actin ring formation (an essential feature of mature osteoclasts induced by RANKL), immunofluorescence staining of F-actin rings was performed. While BMMs cultured with RANKL and M-CSF showed the formation of actin ring structures, as indicated by the rhodamine-phalloidin staining, Rhubarb E inhibited the development of F-actin ring structures in a concentration-dependent manner, as shown in (Fig. 4A, 4B).This result further supports the role of Rhubarb E in inhibiting the RANKL-induced formation of mature osteoclasts.
Fig. 4
Inhibition of F-actin ring formation by Rhubarb E: (A) Immunofluorescence staining of F-actin rings in BMMs treated with Rhubarb E (10, 20, and 50 μg/mL) and stimulated with 100 ng/mL RANKL for 5 days. Regions with red fluorescence represent F-actin rings. (B) Quantitative analysis of TRAP-positive MNC area and cell number. Data were analyzed using oneway ANOVA followed by Tukey’s honest test and are presented as Mean±SD (n=3). Significance levels are inficated as *P≤ 0.05, **P≤0.01.

Discussion
Bone homeostasis is regulated by the balance between bone formation by osteoblasts and bone resorption by osteoclasts [6]. An imbalance between bone formation and bone resorption results in many metabolic bone diseases, including osteosclerosis and osteoporosis [7], which in turn can profoundly impact oral health, contributing to conditions such as periodontal disease and tooth loss [8]. Traditionally, rhubarb has been used to treat constipation, gastrointestinal bleeding, acute pancreatitis, and acute ischemic stroke [4]. In addition, the main active constituents of rhubarb, such as rhein, emodin, and chrysophanol, have a wide range of pharmacological activities with low toxicity and side effects [9]. Recent studies have reported the anticancer, antidiabetic, antimicrobial, and anti-inflammatory effects of rhubarb [10], sugesting its potential as a candidate for the treatment of bone metabolism and inflammatory periodontal diseases.
However, studies investigating the bioactive constituents of rhubarb and their mechanisms in relation to bone health remain largely unexplored. In this study, we found that Rhubarb E inhibited osteoclast differentiation and bone resorption. Moreover, the protective effect of Rhubarb E mainly recapitulated its modulatory effect on the inhibition of osteoclast differentiation.
We demonstrated that Rhubarb E inhibited osteoclast formation in vitro, and this effect was not attributed to the cytotoxicity of Rhubarb E. Upregulation of osteoclast-specific genes such as TRAP, cathepsin K, β-integrin, MMP-9, ATP6V0D2, and DC-STAMP can lead to osteoclast differentiation and activation [11,12]. TRAP, cathepsin K, and NFATc1 are key transcriptional regulators of osteoclastogenesis and can activate the expression of osteoclast-specific genes [13,14]. As expected the gene expression levels of TRAP, cathepsin K, and NFATc1 increased significantly after RANKL treatment but decreased dramatically after Rhubarb E treatment (Fig. 3). These results confirm that Rhubarb E inhibits RANKL-induced osteoclast formation by downregulating the expression of the TRAP, cathepsin K, and NFATc1 genes.
During osteoclastogensis mature osteoclasts are able to form characteristic actin ring structures, which are critical for bone resorption [15,16]. The significant impairment of F-actin ring formation in BMMs treated with Rhubarb E underscores its potential to interfere with osteoclast maturation. The disruption of F-actin ring formation suggests that Rhubarb E not only inhibits osteoclast differentiation but also affects osteoclast maturation.
These results provide consistent support that Rhubarb E modulates periodontitis by regulating osteoclast formation and function. However, further investigation of the efficacy, dosage and safety of Rhubarb E and the mechanisms underlying its effect on osteoclast differentiation are needed for its use within the oral health and dental realm.
Conclusions
This study suggests that Rhubarb E is a potential natural alternative to synthetic drugs for controlling bone loss.
1. Synthetic drugs are often limited by side effects; therefore, harnessing the benefits of natural products such as Rhubarb E is a potentially safer and more effective option.
2. The observed anti-osteoclastic effect was demonstrated in BMMs cultured under RANKL stimulation.
In conclusion, the inhibitory effect on osteoclast differentiation observed in RANKL-stimulated BMMs demonstrates that rhubarb E potentially curb bone loss associated with conditions such as periodontal disease, hence fortifying oral health.



